Recruitment of podocytes from glomerular parietal epithelial cells
- PMID: 19092119
- PMCID: PMC2637040
- DOI: 10.1681/ASN.2008070795
Recruitment of podocytes from glomerular parietal epithelial cells
Abstract
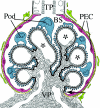
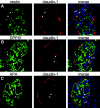
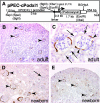
Loss of a critical number of podocytes from the glomerular tuft leads to glomerulosclerosis. Even in health, some podocytes are lost into the urine. Because podocytes themselves cannot regenerate, we postulated that glomerular parietal epithelial cells (PECs), which proliferate throughout life and adjoin podocytes, may migrate to the glomerular tuft and differentiate into podocytes. Here, we describe transitional cells at the glomerular vascular stalk that exhibit features of both PECs and podocytes. Metabolic labeling in juvenile rats suggested that PECs migrate to become podocytes. To prove this, we generated triple-transgenic mice that allowed specific and irreversible labeling of PECs upon administration of doxycycline. PECs were followed in juvenile mice beginning from either postnatal day 5 or after nephrogenesis had ceased at postnatal day 10. In both cases, the number of genetically labeled cells increased over time. All genetically labeled cells coexpressed podocyte marker proteins. In conclusion, we demonstrate for the first time recruitment of podocytes from PECs in juvenile mice. Unraveling the mechanisms of PEC recruitment onto the glomerular tuft may lead to novel therapeutic approaches to renal injury.
Figures





Comment in
-
Parietal epithelial cells regenerate podocytes.J Am Soc Nephrol. 2009 Feb;20(2):231-3. doi: 10.1681/ASN.2008121279. Epub 2009 Jan 28. J Am Soc Nephrol. 2009. PMID: 19176695 No abstract available.
References
-
- US Renal Data System: USRDS 2007 Annual Report, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007
-
- Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 - PubMed
-
- Novick AC, Gephardt G, Guz B, Steinmuller D, Tubbs RR: Long-term follow-up after partial removal of a solitary kidney. N Engl J Med 325: 1058–1062, 1991 - PubMed
-
- Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 - PubMed
-
- Hara M, Yamamoto T, Yanagihara T, Takada T, Itoh M, Adachi Y, Yoshizumi A, Kawasaki K, Kihara I: Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis. Nephron 69: 397–403, 1995 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

