Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02
- PMID: 19093004
- PMCID: PMC2602972
- DOI: 10.1371/journal.pone.0003960
Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02
Abstract
Background: Plasmodium falciparum Apical Membrane Antigen 1 (PfAMA1) is a candidate vaccine antigen expressed by merozoites and sporozoites. It plays a key role in red blood cell and hepatocyte invasion that can be blocked by antibodies.
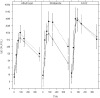
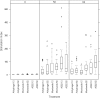
Methodology/principal findings: We assessed the safety and immunogenicity of recombinant PfAMA1 in a dose-escalating, phase Ia trial. PfAMA1 FVO strain, produced in Pichia pastoris, was reconstituted at 10 microg and 50 microg doses with three different adjuvants, Alhydrogel, Montanide ISA720 and AS02 Adjuvant System. Six randomised groups of healthy male volunteers, 8-10 volunteers each, were scheduled to receive three immunisations at 4-week intervals. Safety and immunogenicity data were collected over one year. Transient pain was the predominant injection site reaction (80-100%). Induration occurred in the Montanide 50 microg group, resulting in a sterile abscess in two volunteers. Systemic adverse events occurred mainly in the AS02 groups lasting for 1-2 days. Erythema was observed in 22% of Montanide and 59% of AS02 group volunteers. After the second dose, six volunteers in the AS02 group and one in the Montanide group who reported grade 3 erythema (>50 mm) were withdrawn as they met the stopping criteria. All adverse events resolved. There were no vaccine-related serious adverse events. Humoral responses were highest in the AS02 groups. Antibodies showed activity in an in vitro growth inhibition assay up to 80%. Upon stimulation with the vaccine, peripheral mononuclear cells from all groups proliferated and secreted IFNgamma and IL-5 cytokines.
Conclusions/significance: All formulations showed distinct reactogenicity profiles. All formulations with PfAMA1 were immunogenic and induced functional antibodies.
Trial registration: (Clinicaltrials.gov) NCT00730782.
Conflict of interest statement
Figures




References
-
- Girard MP, Reed ZH, Friede M, Kieny MP. A review of human vaccine research and development: malaria. Vaccine. 2007;25:1567–1580. - PubMed
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–2018. - PubMed
-
- Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, et al. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370:1543–1551. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

