Olig2-induced neural stem cell differentiation involves downregulation of Wnt signaling and induction of Dickkopf-1 expression
- PMID: 19093005
- PMCID: PMC2602983
- DOI: 10.1371/journal.pone.0003917
Olig2-induced neural stem cell differentiation involves downregulation of Wnt signaling and induction of Dickkopf-1 expression
Abstract
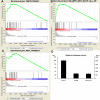
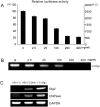
Understanding stem cell-differentiation at the molecular level is important for clinical applications of stem cells and for finding new therapeutic approaches in the context of cancer stem cells. To investigate genome-wide changes involved in differentiation, we have used immortalized neural stem cell (NSC) line (HB1.F3) and Olig2-induced NSC differentiation model (F3.Olig2). Using microarray analysis, we revealed that Olig2-induced NSC differentiation involves downregulation of Wnt pathway, which was further confirmed by TOPflash/FOPflash reporter assay, RT-PCR analysis, immunoblots, and immunocytochemistry. Furthermore, we found that Olig2-induced differentiation induces the expression of Dickkopf-1(Dkk1), a potent antagonist of Wnt signaling. Dkk1 treatment blocked Wnt signaling in HB1.F3 in a dosage-dependent manner, and induced differentiation into astrocytes, oligodendrocytes, and neurons. Our results support cancer stem cell hypothesis which implies that signaling pathway for self-renewal and proliferation of stem cells is maintained till the late stage of differentiation. In our proposed model, Dkk1 may play an important role in downregulating self-renewal and proliferation pathway of stem cells at the late stage of differentiation, and its failure may lead to carcinogenesis.
Conflict of interest statement
Figures




References
-
- Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24:159–171. - PubMed
-
- Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–765. - PubMed
-
- Kilpatrick TJ, Bartlett PF. Cloning and growth of multipotential neural precursors: requirements for proliferation and differentiation. Neuron. 1993;10:255–265. - PubMed
-
- Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989;340:471–473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

