Reprogramming chick RPE progeny cells to differentiate towards retinal neurons by ash1
- PMID: 19093008
- PMCID: PMC2603186
Reprogramming chick RPE progeny cells to differentiate towards retinal neurons by ash1
Abstract
Purpose: Harnessing a cell culture of retinal pigment epithelium (RPE) to give rise to retinal neurons may offer a source of developing neurons for cell-replacement studies. This study explores the possibility of reprogramming RPE progeny cells to differentiate toward retinal neurons with achaete-scute homolog 1 (ash1), a proneural gene that is expressed in progenitor cells in the developing retina and promotes amacrine cell production when overexpressed in the chick retina.
Methods: Replication Competent Avian Splice (RCAS) retrovirus was used to drive the ectopic expression of ash1 in cell cultures of dissociated RPE isolated from day 6 chick embryos. RCAS expressing green fluorescent protein (RCAS-GFP) was used as control. The cultures were examined for de novo generation of neuron-like cells by molecular, cellular, and physiologic criteria.
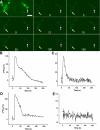
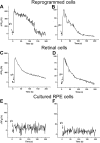
Results: In control cultures infected with RCAS-GFP, RPE cells appeared cobblestone-like and often darkly pigmented. In cultures infected with RCAS-ash1, however, cells remained de-pigmented and frequently formed clusters. Further examination at the morphological and molecular levels showed the development of elaborate processes characteristic of neurons and the expression of genes/markers that identify different types of retinal neurons. The most prevalently expressed neural marker was calretinin, which in the chick retina identifies amacrine, ganglion, and horizontal cells. As an assay for functional maturation, the reprogrammed cells were analyzed for the presence of functional, ionotropic glutamate receptors that lead to a rise in the cytosolic free calcium (Ca(2+)) concentration. Calcium imaging showed that reprogrammed cells responded to glutamate and N-methyl-D-aspartate (NMDA) by increasing their Ca(2+) concentrations, which, after reaching a peak level, returned to the basal level. The response curves of reprogrammed cells resembled those of cultured retinal neurons.
Conclusions: These results suggest that RPE progeny cells can be reprogrammed by ash1 to develop molecular, morphological, and physiologic properties that are characteristic of retinal neurons.
Figures




Similar articles
-
Generating retinal neurons by reprogramming retinal pigment epithelial cells.Expert Opin Biol Ther. 2010 Aug;10(8):1227-39. doi: 10.1517/14712598.2010.495218. Expert Opin Biol Ther. 2010. PMID: 20528097 Free PMC article. Review.
-
Using neurogenin to reprogram chick RPE to produce photoreceptor-like neurons.Invest Ophthalmol Vis Sci. 2010 Jan;51(1):516-25. doi: 10.1167/iovs.09-3822. Epub 2009 Jul 23. Invest Ophthalmol Vis Sci. 2010. PMID: 19628733 Free PMC article.
-
Reprogramming retinal pigment epithelium to differentiate toward retinal neurons with Sox2.Stem Cells. 2009 Jun;27(6):1376-87. doi: 10.1002/stem.48. Stem Cells. 2009. PMID: 19489100 Free PMC article.
-
Proneural gene ash1 promotes amacrine cell production in the chick retina.Dev Neurobiol. 2009 Feb 1-15;69(2-3):88-104. doi: 10.1002/dneu.20693. Dev Neurobiol. 2009. PMID: 19067322 Free PMC article.
-
Using Small Molecules to Reprogram RPE Cells in Regenerative Medicine for Degenerative Eye Disease.Cells. 2024 Nov 21;13(23):1931. doi: 10.3390/cells13231931. Cells. 2024. PMID: 39682681 Free PMC article. Review.
Cited by
-
Neurogenin1 effectively reprograms cultured chick retinal pigment epithelial cells to differentiate toward photoreceptors.J Comp Neurol. 2010 Feb 15;518(4):526-46. doi: 10.1002/cne.22236. J Comp Neurol. 2010. PMID: 20029995 Free PMC article.
-
Augmented Expression of NOGO-A and Its Receptors in Human Retinal Pigment Epithelial Cells Following Treatment with Human Amniotic Fluid.Iran J Public Health. 2022 Jul;51(7):1658-1666. doi: 10.18502/ijph.v51i7.10100. Iran J Public Health. 2022. PMID: 36248281 Free PMC article.
-
Generating retinal neurons by reprogramming retinal pigment epithelial cells.Expert Opin Biol Ther. 2010 Aug;10(8):1227-39. doi: 10.1517/14712598.2010.495218. Expert Opin Biol Ther. 2010. PMID: 20528097 Free PMC article. Review.
-
Direct conversion of adult human retinal pigmented epithelium cells to neurons with photoreceptor properties.Exp Biol Med (Maywood). 2021 Jan;246(2):240-248. doi: 10.1177/1535370220963755. Epub 2020 Oct 18. Exp Biol Med (Maywood). 2021. PMID: 33070653 Free PMC article.
-
Chick retinal pigment epithelium transdifferentiation assay for proneural activities.Methods Mol Biol. 2012;884:201-9. doi: 10.1007/978-1-61779-848-1_14. Methods Mol Biol. 2012. PMID: 22688708 Free PMC article.
References
-
- Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. - PubMed
-
- Cameron DA. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis Neurosci. 2000;17:789–97. - PubMed
-
- Otteson DC, D'Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76. - PubMed
-
- Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
