Clinical and genetic findings in Hungarian patients with X-linked juvenile retinoschisis
- PMID: 19093009
- PMCID: PMC2603250
Clinical and genetic findings in Hungarian patients with X-linked juvenile retinoschisis
Abstract
Purpose: To determine clinical phenotypes, examine the age dependency of X-linked juvenile retinoschisis (XLRS), and identify mutations in the retinoschisis1 gene (RS1) in 13 Hungarian (Caucasian) families with this disease.
Methods: This study included 72 members in 13 families. Complete ophthalmological examinations, including optical coherence tomography (OCT) and full-field and multifocal electroretinography (ERG), were performed on 20 affected males, 13 female carriers, and 27 healthy controls. The patients were divided into two age groups (Group I <25 years and Group II >25 years), retrospectively, to assess the possible effects of age. Correlations among genotype, age, best corrected visual acuity (BCVA), OCT, and ERG results were analyzed. A modified classification scheme was done to identify the different phenotypes of the disease. In each of the 72 family members and 100 age-matched male controls, all exons and introns of RS1 were amplified by polymerase chain reaction (PCR) and directly sequenced.
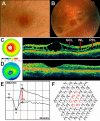
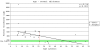
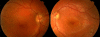
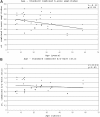
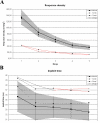
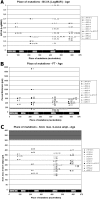
Results: Foveal retinoschisis was detected in 25 eyes (62.5%) of patients by funduscopy, and in 29 eyes (72.5%) by OCT, while macular lamellar schisis was recognizable only by OCT in 30 eyes (75%) of patients. Foveal thickness (FT) and total macular volume were significantly increased in younger (Group I) patients only. For patients younger than 26 years, large inner nuclear central cysts were observable by OCT, while after 26 years, foveas were atrophic. White flecks and dots, which were like that seen in fundus albipunctatus, were detected in both eyes of one patient. In both patient groups, characteristically decreased b-waves of standard combined ERG were recorded without any significant difference between the patient groups. The BCVA and ERG parameters of all patients and the OCT of younger patients were significantly worse (p<0.05) than those of age-matched controls. A significant difference between the two age groups was found in case FT, total macular volume, and amplitudes of rod b-wave only. Moderate negative correlation (r=-0.54, p<0.001) was detected between age and FT, while only low negative correlation (r=-0.33, p<0.05) was detected between age and standard combined b-wave amplitudes of full-field ERG. BCVA LogMAR did not show any obvious correlation with age (r=-0.14, p=0.39) or with the type of mutation. Nine different mutations were identified in 25 male patients and 31 female carriers of 13 families: six known and one novel missense mutation (c.575C>T, p.Pro192Leu), one insertion mutation (c.579dupC, p.Ile194Hisfs29ext43), and one frameshift, causing splice site mutation (c.78+1G>C) were detected. These mutations were absent in the 100 age-matched male control samples.
Conclusions: Foveal cystic schisis was found more often by OCT than by funduscopy (+10%), while flat macular lamellar schisis was recognizable only by OCT. Advancing age inversely influenced the size of cavities (FT), and standard combined b-wave amplitudes of full-field ERG, while BCVA, response density, and implicit times of multifocal electroretinography did not show any obvious correlation with age. The atrophic stage of the disease was observable after 26 years of age. The lesions that appeared to be indicative of fundus albipunctatus were proven to be palisades between the splitted retinal layers. Our modified classification scheme was helpful in assessing the prevalence of disease types. In these Hungarian patients, one novel and eight known mutations were detected. The distribution of mutations in RS1 was different to that reported in the literature, because the greatest number of different mutations was in exon 6 instead of exon 4. Two mutation hot spots were found: between c.418-422 in exon 5 and between c.574-579 in exon 6. Genotype-phenotype correlation was not demonstrable.
Figures


Similar articles
-
Truncation of retinoschisin protein associated with a novel splice site mutation in the RS1 gene.Mol Vis. 2008 Aug 25;14:1549-58. Mol Vis. 2008. PMID: 18728755 Free PMC article.
-
Abnormal cone structure in foveal schisis cavities in X-linked retinoschisis from mutations in exon 6 of the RS1 gene.Invest Ophthalmol Vis Sci. 2011 Dec 20;52(13):9614-23. doi: 10.1167/iovs.11-8600. Invest Ophthalmol Vis Sci. 2011. PMID: 22110067 Free PMC article.
-
Phenotypic characterization of X-linked retinoschisis: Clinical, electroretinography, and optical coherence tomography variables.Indian J Ophthalmol. 2016 Jul;64(7):513-7. doi: 10.4103/0301-4738.190140. Indian J Ophthalmol. 2016. PMID: 27609164 Free PMC article.
-
Of men and mice: Human X-linked retinoschisis and fidelity in mouse modeling.Prog Retin Eye Res. 2022 Mar;87:100999. doi: 10.1016/j.preteyeres.2021.100999. Epub 2021 Aug 11. Prog Retin Eye Res. 2022. PMID: 34390869 Review.
-
Review: Clinical findings and genetic characterization of children affected with X-linked retinoschisis in the Spanish population.Eur J Ophthalmol. 2025 May;35(3):809-820. doi: 10.1177/11206721241305244. Epub 2024 Dec 8. Eur J Ophthalmol. 2025. PMID: 39648411 Review.
Cited by
-
Clinical observations of vitreoretinal surgery for four different phenotypes of X-linked congenital retinoschisis.Int J Ophthalmol. 2018 Jun 18;11(6):986-990. doi: 10.18240/ijo.2018.06.15. eCollection 2018. Int J Ophthalmol. 2018. PMID: 29977812 Free PMC article.
-
Long-term rearrangement of retinal structures in a novel mutation of X-linked retinoschisis.Biomed Rep. 2017 Sep;7(3):241-246. doi: 10.3892/br.2017.954. Epub 2017 Jul 27. Biomed Rep. 2017. PMID: 28811895 Free PMC article.
-
A retrospective longitudinal study of 52 Finnish patients with X-linked retinoschisis.Acta Ophthalmol. 2025 Mar;103(2):196-204. doi: 10.1111/aos.16776. Epub 2024 Oct 22. Acta Ophthalmol. 2025. PMID: 39435478 Free PMC article.
-
Novel RS1 mutations associated with X-linked juvenile retinoschisis.Int J Mol Med. 2012 Apr;29(4):644-8. doi: 10.3892/ijmm.2012.882. Epub 2012 Jan 10. Int J Mol Med. 2012. PMID: 22245991 Free PMC article.
-
Long-term 12 year follow-up of X-linked congenital retinoschisis.Ophthalmic Genet. 2010 Sep;31(3):114-25. doi: 10.3109/13816810.2010.482555. Ophthalmic Genet. 2010. PMID: 20569020 Free PMC article.
References
-
- Mooy CM, Van Den Born LI, Baarsma S, Paridaens DA, Kraaijenbrink T, Bergen A, Weber BH. Hereditary X-linked juvenile retinoschisis: a review of the role of Müller cells. Arch Ophthalmol. 2002;120:979–84. - PubMed
-
- Hiriyanna K, Singh-Parikshak R, Bingham EL, Kemp JA, Ayyagari R, Yashar BM, Sieving PA. Searching for genotype-phenotype correlations in X-linked juvenile retinoschisis. In: Anderson RE, La Vail MM, Hollyfield JG, eds. New Insights into Retinal Degenerative Diseases. New York: Plenum Publishers; 2001;45–53.
-
- Wang T, Waters CT, Rothman AM, Jakins TJ, Römisch K, Trump D. Intracellular retention of mutant retinoschisin is the pathological mechanism underlaying X-linked retinoschisis. Hum Mol Genet. 2002;11:3097–105. - PubMed
-
- Tantri A, Vrabec TR, Cu-Unjieng A, Frost A, Annesley WH, Jr, Donoso LA. X-linked retinoschisis: A clinical and molecular genetic review. Surv Ophthalmol. 2004;49:214–30. - PubMed
-
- McKibbin M, Booth AP, George NDL. Foveal ectopia in X-linked retinoschisis. Retina. 2001;21:361–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
