Zebrafish myelination: a transparent model for remyelination?
- PMID: 19093028
- PMCID: PMC2590821
- DOI: 10.1242/dmm.001248
Zebrafish myelination: a transparent model for remyelination?
Abstract
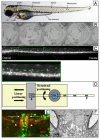
There is currently an unmet need for a therapy that promotes the regenerative process of remyelination in central nervous system diseases, notably multiple sclerosis (MS). A high-throughput model is, therefore, required to screen potential therapeutic drugs and to refine genomic and proteomic data from MS lesions. Here, we review the value of the zebrafish (Danio rerio) larva as a model of the developmental process of myelination, describing the powerful applications of zebrafish for genetic manipulation and genetic screens, as well as some of the exciting imaging capabilities of this model. Finally, we discuss how a model of zebrafish myelination can be used as a high-throughput screening model to predict the effect of compounds on remyelination. We conclude that zebrafish provide a highly versatile myelination model. As more complex transgenic zebrafish lines are developed, it might soon be possible to visualise myelination, or even remyelination, in real time. However, experimental outputs must be designed carefully for such visual and temporal techniques.
Figures
Similar articles
-
Knockdown of Lingo1b protein promotes myelination and oligodendrocyte differentiation in zebrafish.Exp Neurol. 2014 Jan;251:72-83. doi: 10.1016/j.expneurol.2013.11.012. Epub 2013 Nov 18. Exp Neurol. 2014. PMID: 24262204
-
A novel myelin protein zero transgenic zebrafish designed for rapid readout of in vivo myelination.Glia. 2019 Apr;67(4):650-667. doi: 10.1002/glia.23559. Epub 2019 Jan 9. Glia. 2019. PMID: 30623975 Free PMC article.
-
Models for Studying Myelination, Demyelination and Remyelination.Neuromolecular Med. 2017 Sep;19(2-3):181-192. doi: 10.1007/s12017-017-8442-1. Epub 2017 May 23. Neuromolecular Med. 2017. PMID: 28536996 Review.
-
Drug reprofiling using zebrafish identifies novel compounds with potential pro-myelination effects.Neuropharmacology. 2010 Sep;59(3):149-59. doi: 10.1016/j.neuropharm.2010.04.014. Epub 2010 May 5. Neuropharmacology. 2010. PMID: 20450924
-
Insights into mechanisms of central nervous system myelination using zebrafish.Glia. 2016 Mar;64(3):333-49. doi: 10.1002/glia.22897. Epub 2015 Aug 6. Glia. 2016. PMID: 26250418 Review.
Cited by
-
An automated high-resolution in vivo screen in zebrafish to identify chemical regulators of myelination.Elife. 2018 Jul 6;7:e35136. doi: 10.7554/eLife.35136. Elife. 2018. PMID: 29979149 Free PMC article.
-
EXOSC8 mutations alter mRNA metabolism and cause hypomyelination with spinal muscular atrophy and cerebellar hypoplasia.Nat Commun. 2014 Jul 3;5:4287. doi: 10.1038/ncomms5287. Nat Commun. 2014. PMID: 24989451 Free PMC article.
-
Zebrafish as a model to investigate CNS myelination.Glia. 2015 Feb;63(2):177-93. doi: 10.1002/glia.22755. Epub 2014 Sep 27. Glia. 2015. PMID: 25263121 Free PMC article. Review.
-
SCORE imaging: specimen in a corrected optical rotational enclosure.Zebrafish. 2010 Jun;7(2):149-54. doi: 10.1089/zeb.2010.0660. Zebrafish. 2010. PMID: 20528262 Free PMC article.
-
Lysophosphatidic acid signaling via LPA6 : A negative modulator of developmental oligodendrocyte maturation.J Neurochem. 2022 Dec;163(6):478-499. doi: 10.1111/jnc.15696. Epub 2022 Oct 18. J Neurochem. 2022. PMID: 36153691 Free PMC article.
References
-
- Altmann D. M., Boyton R. J. (2004). Models of multiple sclerosis. Drug Discov. Today Dis. Models 1, 405–410
-
- Becker T., Wullimann M. F., Becker C. G., Bernhardt R. R., Schachner M. (1997). Axonal regrowth after spinal cord transection in adult zebrafish. J. Comp. Neurol. 377, 577–595 - PubMed
-
- Berger J., Currie P. (2007). The role of zebrafish in chemical genetics. Curr. Med. Chem. 14, 2413–2420 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

