Peptidylarginine deiminase 2 (PAD2) overexpression in transgenic mice leads to myelin loss in the central nervous system
- PMID: 19093029
- PMCID: PMC2590822
- DOI: 10.1242/dmm.000729
Peptidylarginine deiminase 2 (PAD2) overexpression in transgenic mice leads to myelin loss in the central nervous system
Abstract
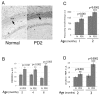
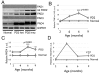
Demyelination in the central nervous system is the hallmark feature in multiple sclerosis (MS). The mechanism resulting in destabilization of myelin is a complex multi-faceted process, part of which involves deimination of myelin basic protein (MBP). Deimination, the conversion of protein-bound arginine to citrulline, is mediated by the peptidylarginine deiminase (PAD) family of enzymes, of which the PAD2 and PAD4 isoforms are present in myelin. To test the hypothesis that PAD contributes to destabilization of myelin in MS, we developed a transgenic mouse line (PD2) containing multiple copies of the cDNA encoding PAD2, under the control of the MBP promoter. Using previously established criteria, clinical signs were more severe in PD2 mice than in their normal littermates. The increase in PAD2 expression and activity in white matter was demonstrated by immunohistochemistry, reverse transcriptase-PCR, enzyme activity assays, and increased deimination of MBP. Light and electron microscopy revealed more severe focal demyelination and thinner myelin in the PD2 homozygous mice compared with heterozygous PD2 mice. Quantitation of the disease-associated molecules GFAP and CD68, as measured by immunoslot blots, were indicative of astrocytosis and macrophage activation. Concurrently, elevated levels of the pro-inflammatory cytokine TNF-alpha and nuclear histone deimination support initiation of demyelination by increased PAD activity. These data support the hypothesis that elevated PAD levels in white matter represents an early change that precedes demyelination.
Figures






Similar articles
-
Myelin localization of peptidylarginine deiminases 2 and 4: comparison of PAD2 and PAD4 activities.Lab Invest. 2008 Apr;88(4):354-64. doi: 10.1038/labinvest.3700748. Epub 2008 Jan 28. Lab Invest. 2008. PMID: 18227806
-
Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation.J Neurosci. 2006 Nov 1;26(44):11387-96. doi: 10.1523/JNEUROSCI.3349-06.2006. J Neurosci. 2006. PMID: 17079667 Free PMC article.
-
Peptidylarginine deiminase: a candidate factor in demyelinating disease.J Neurochem. 2002 Apr;81(2):335-43. doi: 10.1046/j.1471-4159.2002.00834.x. J Neurochem. 2002. PMID: 12064481
-
Myelin Basic Protein Citrullination in Multiple Sclerosis: A Potential Therapeutic Target for the Pathology.Neurochem Res. 2016 Aug;41(8):1845-56. doi: 10.1007/s11064-016-1920-2. Epub 2016 Apr 21. Neurochem Res. 2016. PMID: 27097548 Review.
-
Retinal deimination in aging and disease.IUBMB Life. 2009 May;61(5):504-9. doi: 10.1002/iub.184. IUBMB Life. 2009. PMID: 19391158 Review.
Cited by
-
Peptidylarginine deiminase and protein citrullination in prion diseases: strong evidence of neurodegeneration.Prion. 2013 Jan-Feb;7(1):42-6. doi: 10.4161/pri.22380. Epub 2012 Sep 28. Prion. 2013. PMID: 23022892 Free PMC article. Review.
-
Potential role for PAD2 in gene regulation in breast cancer cells.PLoS One. 2012;7(7):e41242. doi: 10.1371/journal.pone.0041242. Epub 2012 Jul 24. PLoS One. 2012. PMID: 22911765 Free PMC article.
-
Identifying mRNAs Residing in Myelinating Oligodendrocyte Processes as a Basis for Understanding Internode Autonomy.Life (Basel). 2023 Apr 4;13(4):945. doi: 10.3390/life13040945. Life (Basel). 2023. PMID: 37109474 Free PMC article.
-
Identification of Padi2 as a novel angiogenesis-regulating gene by genome association studies in mice.PLoS Genet. 2017 Jun 15;13(6):e1006848. doi: 10.1371/journal.pgen.1006848. eCollection 2017 Jun. PLoS Genet. 2017. PMID: 28617813 Free PMC article.
-
Classical 18.5-and 21.5-kDa isoforms of myelin basic protein inhibit calcium influx into oligodendroglial cells, in contrast to golli isoforms.J Neurosci Res. 2011 Apr;89(4):467-80. doi: 10.1002/jnr.22570. Epub 2011 Jan 13. J Neurosci Res. 2011. PMID: 21312222 Free PMC article.
References
-
- Akassoglou K., Bauer J., Kassiotis G., Pasparakis M., Lassmann H., Kollias G., Probert L. (1998)Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am. J. Pathol. 153, 801–813 - PMC - PubMed
-
- Akassoglou K., Bauer J., Kassiotis G., Lassmann H., Kollias G., Probert L. (1999)Transgenic models of TNF induced demyelination. Adv. Exp. Med. Biol. 468, 245–259 - PubMed
-
- Bruck W. (2007)New Insights into the pathology of multiple sclerosis: towards a unified concept. J. Neurol. 254, 3–9
-
- Chung I. Y., Benveniste E. N. (1990)Tumor necrosis factor-alpha production by astrocytes: induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J. Immunol. 144, 2999–3007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

