Analysis of mouse models carrying the I26T and R160C substitutions in the transcriptional repressor HESX1 as models for septo-optic dysplasia and hypopituitarism
- PMID: 19093031
- PMCID: PMC2590837
- DOI: 10.1242/dmm.000711
Analysis of mouse models carrying the I26T and R160C substitutions in the transcriptional repressor HESX1 as models for septo-optic dysplasia and hypopituitarism
Abstract
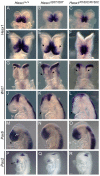
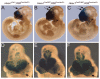
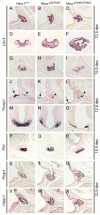
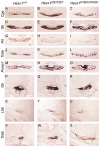
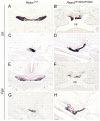
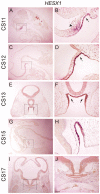
A homozygous substitution of the highly conserved isoleucine at position 26 by threonine (I26T) in the transcriptional repressor HESX1 has been associated with anterior pituitary hypoplasia in a human patient, with no forebrain or eye defects. Two individuals carrying a homozygous substitution of the conserved arginine at position 160 by cysteine (R160C) manifest septo-optic dysplasia (SOD), a condition characterised by pituitary abnormalities associated with midline telencephalic structure defects and optic nerve hypoplasia. We have generated two knock-in mouse models containing either the I26T or R160C substitution in the genomic locus. Hesx1(I26T/I26T) embryos show pituitary defects comparable with Hesx1(-/-) mouse mutants, with frequent occurrence of ocular abnormalities, although the telencephalon develops normally. Hesx1(R160C/R160C) mutants display forebrain and pituitary defects that are identical to those observed in Hesx1(-/-) null mice. We also show that the expression pattern of HESX1 during early human development is very similar to that described in the mouse, suggesting that the function of HESX1 is conserved between the two species. Together, these results suggest that the I26T mutation yields a hypomorphic allele, whereas R160C produces a null allele and, consequently, a more severe phenotype in both mice and humans.
Figures



Similar articles
-
Molecular effects of novel mutations in Hesx1/HESX1 associated with human pituitary disorders.Development. 2001 Dec;128(24):5189-99. doi: 10.1242/dev.128.24.5189. Development. 2001. PMID: 11748154
-
A homozygous mutation in HESX1 is associated with evolving hypopituitarism due to impaired repressor-corepressor interaction.J Clin Invest. 2003 Oct;112(8):1192-201. doi: 10.1172/JCI18589. J Clin Invest. 2003. PMID: 14561704 Free PMC article.
-
HESX1 mutations in patients with congenital hypopituitarism: variable phenotypes with the same genotype.Clin Endocrinol (Oxf). 2016 Sep;85(3):408-14. doi: 10.1111/cen.13067. Epub 2016 Apr 28. Clin Endocrinol (Oxf). 2016. PMID: 27000987 Free PMC article.
-
HESX1: a novel gene implicated in a familial form of septo-optic dysplasia.Acta Paediatr Suppl. 1999 Dec;88(433):49-54. doi: 10.1111/j.1651-2227.1999.tb14403.x. Acta Paediatr Suppl. 1999. PMID: 10626545 Review.
-
Septo-optic dysplasia and other midline defects: the role of transcription factors: HESX1 and beyond.Best Pract Res Clin Endocrinol Metab. 2011 Feb;25(1):115-24. doi: 10.1016/j.beem.2010.06.008. Best Pract Res Clin Endocrinol Metab. 2011. PMID: 21396578 Review.
Cited by
-
Developmental analysis and influence of genetic background on the Lhx3 W227ter mouse model of combined pituitary hormone deficiency disease.Endocrinology. 2013 Feb;154(2):738-48. doi: 10.1210/en.2012-1790. Epub 2013 Jan 3. Endocrinology. 2013. PMID: 23288907 Free PMC article.
-
Genetics of Combined Pituitary Hormone Deficiency: Roadmap into the Genome Era.Endocr Rev. 2016 Dec;37(6):636-675. doi: 10.1210/er.2016-1101. Epub 2016 Nov 9. Endocr Rev. 2016. PMID: 27828722 Free PMC article. Review.
-
The molecular basis of hypopituitarism.Trends Endocrinol Metab. 2009 Dec;20(10):506-16. doi: 10.1016/j.tem.2009.06.005. Epub 2009 Oct 23. Trends Endocrinol Metab. 2009. PMID: 19854060 Free PMC article. Review.
-
Recent advances in central congenital hypothyroidism.J Endocrinol. 2015 Dec;227(3):R51-71. doi: 10.1530/JOE-15-0341. Epub 2015 Sep 28. J Endocrinol. 2015. PMID: 26416826 Free PMC article. Review.
-
Hypothalamic sonic hedgehog is required for cell specification and proliferation of LHX3/LHX4 pituitary embryonic precursors.Development. 2017 Sep 15;144(18):3289-3302. doi: 10.1242/dev.153387. Epub 2017 Aug 14. Development. 2017. PMID: 28807898 Free PMC article.
References
-
- Allen T., Lobe C. G. (1999)A comparison of Notch, Hes and Grg expression during murine embryonic and post-natal development. Cell Mol. Biol. 45, 687–708 - PubMed
-
- Brickman J. M., Clements M., Tyrell R., McNay D., Woods K., Warner J., Stewart A., Beddington R. S. P., Dattani M. (2001)Molecular effects of novel mutations in Hesx1/HESX1 associated with human pituitary disorders. Development 128, 5189–5199 - PubMed
-
- Carvalho L. R., Woods K. S., Mendonca B. B., Marcal N., Zamparini A. L., Stifani S., Brickman J. M., Arnhold I. J., Dattani M. T. (2003)A homozygous mutation in HESX1 is associated with evolving hypopituitarism due to impaired repressor-corepressor interaction. J. Clin. Invest. 112, 1192–1201 - PMC - PubMed
-
- Cha K. B., Douglas K. R., Potok M. A., Liang H., Jones S. N., Camper S. A. (2004)WNT5A signaling affects pituitary gland shape. Mech. Dev. 121, 183–194 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

