Development of the human Achilles tendon enthesis organ
- PMID: 19094187
- PMCID: PMC2666140
- DOI: 10.1111/j.1469-7580.2008.00997.x
Development of the human Achilles tendon enthesis organ
Abstract
The attachment of the Achilles tendon is part of an 'enthesis organ' that reduces stress concentration at the hard-soft tissue interface. The organ also includes opposing sesamoid and periosteal fibrocartilages, a bursa and Kager's fat pad. In addition, the deep crural and plantar fasciae contribute to Achilles stress dissipation and could also be regarded as components. Here we describe the sequence in which these various tissues differentiate. Serial sections of feet from spontaneously aborted foetuses (crown rump lengths 22-322 mm) were examined. All slides formed part of an existing collection of histologically sectioned embryological material, obtained under Spanish law and housed in the Universidad Complutense, Madrid. From the earliest stages, it was evident that the Achilles tendon and plantar fascia had a mutual attachment to the calcaneal perichondrium. The first components of the enthesis organ to appear (in the 45-mm foetus) were the retrocalcaneal bursa and the crural fascia. The former developed by cavitation within the mesenchyme that later gave rise to Kager's fat pad. The tip of the putative fat pad protruded into the developing bursa in the 110-mm foetus and fully differentiated adipocytes were apparent in the 17-mm foetus. All three fibrocartilages were first recognisable in the 332-mm foetus--at which time adipogenesis had commenced in the heel fat pad. The sequence in which the various elements became apparent suggests that bursal formation and the appearance of the crural fascia may be necessary to facilitate the foot movements that subsequently lead to fibrocartilage differentiation. The later commencement of adipogenesis in the heel than in Kager's pad probably reflects the non-weight environment in utero. The direct continuity between plantar fascia and Achilles tendon that is characteristic of the adult reflects the initial attachment of both structures to the calcaneal perichondrium rather than to the skeletal anlagen itself.
Figures

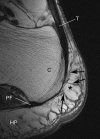


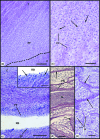
References
-
- Andersen H. Histochemistry and development of the human shoulder and acromioclavicular joints with particular reference to the early development of the clavicle. Acta Anat (Basel) 1963;55:124–165. - PubMed
-
- Andersen H, Bro-Rasmussen F. Histochemical studies on the histogenesis of the joints in human fetuses with special reference to the development of the joint cavities in the hand and foot. Am J Anat. 1961;108:111–122.
-
- Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69:144–155. - PubMed
-
- Benjamin M, McGonagle D. Histopathologic changes at ‘synovio–entheseal complexes’ suggesting a novel mechanism for synovitis in osteoarthritis and spondylarthritis. Arthritis Rheum. 2007;56:3601–3609. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

