Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells
- PMID: 19094203
- PMCID: PMC2627907
- DOI: 10.1186/1471-2180-8-229
Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells
Abstract
Background: Mannose binding lectin (MBL) is an important host defence protein against opportunistic fungal pathogens. This carbohydrate-binding protein, an opsonin and lectin pathway activator, binds through multiple lectin domains to the repeating sugar arrays displayed on the surface of a wide range of clinically relevant microbial species. We investigated the contribution of MBL to antifungal innate immunity towards C. parapsilosis in vitro.
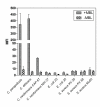
Results: High avidity binding was observed between MBL and C. albicans and C. parapsilosis. Addition of MBL to MBL deficient serum increased the deposition of C4 and C3b and enhanced the uptake of C. albicans, C. parapsilosis and acapsular C. neoformans by polymorphonuclear cells (PMNs). Compared to other microorganisms, such as Escherichia coli, Staphylococcus aureus and Cryptococcus neoformans, C. parapsilosis and Candida albicans were potent activators of the lectin pathway.
Conclusion: Our results suggest that MBL plays a crucial role in the innate immunity against infections caused by yeast by increasing uptake by PMN.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

