Isolation of antibodies specific to a single conformation-dependant antigenic determinant on the EG95 hydatid vaccine
- PMID: 19095030
- PMCID: PMC2670974
- DOI: 10.1016/j.vaccine.2008.11.096
Isolation of antibodies specific to a single conformation-dependant antigenic determinant on the EG95 hydatid vaccine
Abstract
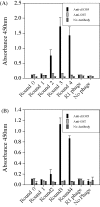
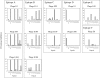
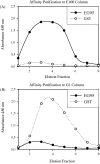
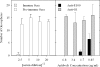
EG95 is a recombinant vaccine protein that elicits protection against hydatid disease in sheep. Previous studies have shown that the host-protective epitopes on EG95 depend on correct conformation and cannot be represented by simple "linear" peptides. By screening random peptide phage display libraries with polyclonal antibodies directed against conformation-dependant epitopes of EG95, we have selected a number of peptides that mimic these epitopes. The selected peptides did not show sequence homology to EG95. Antigen binding assays involving these peptides have provided evidence of at least four conformationally-dependant epitope regions on EG95. One of the selected peptides, E100, has been used to purify antibodies from anti-sera raised in sheep vaccinated with EG95. This yielded monospecific antibodies capable of recognizing recombinant EG95 in ELISA and native EG95 in Western blot assays. This antibody was demonstrated to be effective in antibody-dependant complement-mediated in vitro killing of Echinococcus granulosus oncospheres. Peptide E100 may represent the basis for a quality control assay for EG95 production, and has the potential to become a component of a synthetic peptide-based vaccine against E. granulosus.
Figures




References
-
- Thompson R.C.A., Allsopp C.E. C.A.B. International; Wallingford: 1988. Hydatidosis: veterinary perspectives and annotated bibliography.
-
- Lightowlers M.W., Lawrence S.B., Gauci C.G., Young J., Ralston M.J., Maas D. Vaccination against hydatidosis using a defined recombinant antigen. Parasite Immunol. 1996;18(9):457–462. - PubMed
-
- Lightowlers M.W., Jensen O., Fernandez E., Iriarte J.A., Woollard D.J., Gauci C.G. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int J Parasitol. 1999;29(4):531–534. - PubMed
-
- Lightowlers M.W., Colebrook A.L., Gauci C.G., Gauci S.M., Kyngdon C.T., Monkhouse J.L. Vaccination against cestode parasites: anti-helminth vaccines that work and why. Vet Parasitol. 2003;115(2):83–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

