Fast reversed-phase liquid chromatography to reduce back exchange and increase throughput in H/D exchange monitored by FT-ICR mass spectrometry
- PMID: 19095461
- PMCID: PMC2673454
- DOI: 10.1016/j.jasms.2008.11.010
Fast reversed-phase liquid chromatography to reduce back exchange and increase throughput in H/D exchange monitored by FT-ICR mass spectrometry
Abstract
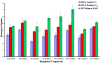
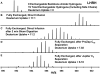
In solution-phase hydrogen/deuterium exchange (HDX), it is essential to minimize the back-exchange level of H for D after the exchange has been quenched, to accurately assign protein conformation and protein-protein or protein-ligand interactions. Reversed-phase HPLC is conducted at low pH and low temperature to desalt and separate proteolytic fragments. However, back exchange averages roughly 30% because of the long exposure to H(2)O in the mobile phase. In this report, we first show that there is no significant backbone amide hydrogen back exchange during quench and digestion; backbone exchange occurs primarily during subsequent liquid chromatography separation. We then show that a rapid reversed-phase separation reduces back exchange for HDX by at least 25%, resulting from the dramatically reduced retention time of the peptide fragments on the column. The influence of retention time on back exchange was also evaluated. The rapid separation coupled with high-resolution FT-ICR MS at 14.5 T provides high amino acid sequence coverage, high sample throughput, and high reproducibility and reliability.
Figures

References
-
- Engen JR, Smith DL. Investigating Protein Structure and Dynamics by Hydrogen Exchange MS. Anal Chem. 2001;73:256A–265A. - PubMed
-
- Lam TT, Lanman JK, Emmett ER, Hendrickson CL, Marshall AG, Prevelige PE. Mapping of Protein:Protein Contact Surfaces by Hydrogen/Deuterium Exchange, Followed by On-line High-Performance Liquid chromatography-Electrospray Ionization Fourier-Transform Ion-Cyclotron-Resonance Mass Analysis. Journal of Chromatography A. 2002;982:85–95. - PubMed
-
- Wales TE, Engen JR. Hydrogen Exchange Mass Spectrometry for the Analysis of Protein Dynamics. Mass Spectrometry Reviews. 2006;25:158–170. - PubMed
-
- Kipping M, Simmons DA. Protein-folding Kinetics and Mechanisms Studied by Pulsed-labeling and Mass Spectrometry. J Mass Spectrom. 2003;38:271–276.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

