Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals
- PMID: 19095642
- PMCID: PMC2643501
- DOI: 10.1074/jbc.M808798200
Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals
Abstract
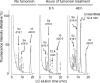
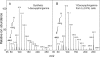
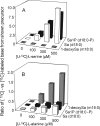
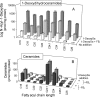
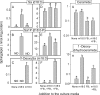
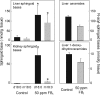
Fumonisin B(1) (FB(1)) is a mycotoxin that inhibits ceramide synthases (CerS) and causes kidney and liver toxicity and other disease. Inhibition of CerS by FB(1) increases sphinganine (Sa), Sa 1-phosphate, and a previously unidentified metabolite. Analysis of the latter by quadrupole-time-of-flight mass spectrometry assigned an m/z = 286.3123 in positive ionization mode, consistent with the molecular formula for deoxysphinganine (C(18)H(40)NO). Comparison with a synthetic standard using liquid chromatography, electrospray tandem mass spectrometry identified the metabolite as 1-deoxysphinganine (1-deoxySa) based on LC mobility and production of a distinctive fragment ion (m/z 44, CH(3)CH=NH (+)(2)) upon collision-induced dissociation. This novel sphingoid base arises from condensation of alanine with palmitoyl-CoA via serine palmitoyltransferase (SPT), as indicated by incorporation of l-[U-(13)C]alanine into 1-deoxySa by Vero cells; inhibition of its production in LLC-PK(1) cells by myriocin, an SPT inhibitor; and the absence of incorporation of [U-(13)C]palmitate into 1-[(13)C]deoxySa in LY-B cells, which lack SPT activity. LY-B-LCB1 cells, in which SPT has been restored by stable transfection, however, produce large amounts of 1-[(13)C]deoxySa. 1-DeoxySa was elevated in FB(1)-treated cells and mouse liver and kidney, and its cytotoxicity was greater than or equal to that of Sa for LLC-PK(1) and DU-145 cells. Therefore, this compound is likely to contribute to pathologies associated with fumonisins. In the absence of FB(1), substantial amounts of 1-deoxySa are made and acylated to N-acyl-1-deoxySa (i.e. 1-deoxydihydroceramides). Thus, these compounds are an underappreciated category of bioactive sphingoid bases and "ceramides" that might play important roles in cell regulation.
Figures


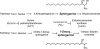
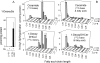

References
-
- Marasas, W. F. O., Riley, R. T., Hendricks, K. A., Stevens, V. L., Sadler, T. W., Gelineau-van Waes, J. B., Missmer, S. A., Cabrera, J., Torres, O., Gelderblom, W. C. A., Allgood, J., Martinez, C., Maddox, J., Miller, J. D., Starr, L., Sullards, M. C., Roman, A. V., Voss, K. A., Wang, E., and Merrill, A. H., Jr. (2004) J. Nutr. 134 711–716 - PubMed
-
- NTP Technical Report (2002). NIH Publication No. 99-3955
-
- Wang, E., Norred, W. P., Bacon, C. W., Riley, R. T., and Merrill, A. H., Jr. (1991) J. Biol. Chem. 266 14486–14490 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

