Estrogen-mediated suppression of the gene encoding protein tyrosine phosphatase PTPRO in human breast cancer: mechanism and role in tamoxifen sensitivity
- PMID: 19095770
- PMCID: PMC2646619
- DOI: 10.1210/me.2008-0211
Estrogen-mediated suppression of the gene encoding protein tyrosine phosphatase PTPRO in human breast cancer: mechanism and role in tamoxifen sensitivity
Abstract
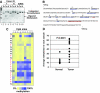
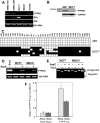
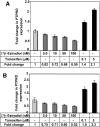
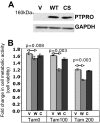
We have previously demonstrated the tumor suppressor characteristics of protein tyrosine phosphatase receptor-type O (PTPRO) in leukemia and lung cancer, including its suppression by promoter methylation. Here, we show tumor-specific methylation of the PTPRO CpG island in primary human breast cancer. PTPRO expression was significantly reduced in established breast cancer cell lines MCF-7 and MDA-MB-231 due to promoter methylation compared with its expression in normal human mammary epithelial cells (48R and 184). Further, the silenced gene could be demethylated and reactivated in MCF-7 and MDA-MB-231 cells upon treatment with 5-Azacytidine, a DNA hypomethylating agent. Because PTPRO promoter harbors estrogen-responsive elements and 17beta-estradiol (E2) plays a role in breast carcinogenesis, we examined the effect of E2 and its antagonist tamoxifen on PTPRO expression in human mammary epithelial cells and PTPRO-expressing breast cancer cell line Hs578t. Treatment with E2 significantly curtailed PTPRO expression in 48R and Hs578t cells, which was facilitated by ectopic expression of estrogen receptor (ER)beta but not ERalpha. On the contrary, treatment with tamoxifen increased PTPRO expression. Further, knockdown of ERbeta by small interfering RNA abolished these effects of E2 and tamoxifen. Chromatin immunoprecipitation assay showed association of c-Fos and c-Jun with PTPRO promoter in untreated cells, which was augmented by tamoxifen-mediated recruitment of ERbeta to the promoter. Estradiol treatment resulted in dissociation of c-Fos and c-Jun from the promoter. Ectopic expression of PTPRO in the nonexpressing MCF-7 cells sensitized them to growth-suppressive effects of tamoxifen. These data suggest that estrogen-mediated suppression of PTPRO is probably one of the early events in estrogen-induced tumorigenesis and that expression of PTPRO could facilitate endocrine therapy of breast cancer.
Figures




References
-
- Russo J, Hu YF, Yang X, Russo IH 2000 Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr:17–37 - PubMed
-
- Peppercorn J, Perou CM, Carey LA 2008 Molecular subtypes in breast cancer evaluation and management: divide and conquer. Cancer Invest 26:1–10 - PubMed
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D 2000 Molecular portraits of human breast tumours. Nature 406:747–752 - PubMed
-
- Henderson BE, Feigelson HS 2000 Hormonal carcinogenesis. Carcinogenesis 21:427–433 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

