The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors
- PMID: 19095792
- PMCID: PMC2605416
- DOI: 10.1073/pnas.0805408105
The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors
Abstract
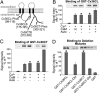
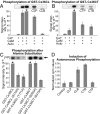
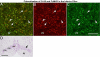
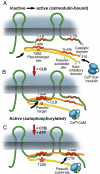
Electrical synapses can undergo activity-dependent plasticity. The calcium/calmodulin-dependent kinase II (CaMKII) appears to play a critical role in this phenomenon, but the underlying mechanisms of how CaMKII affects the neuronal gap junction protein connexin36 (Cx36) are unknown. Here we demonstrate effective binding of (35)S-labeled CaMKII to 2 juxtamembrane cytoplasmic domains of Cx36 and in vitro phosphorylation of this protein by the kinase. Both domains reveal striking similarities with segments of the regulatory subunit of CaMKII, which include the pseudosubstrate and pseudotarget sites of the kinase. Similar to the NR2B subunit of the NMDA receptor both Cx36 binding sites exhibit phosphorylation-dependent interaction and autonomous activation of CaMKII. CaMKII and Cx36 were shown to be significantly colocalized in the inferior olive, a brainstem nucleus highly enriched in electrical synapses, indicating physical proximity of these proteins. In analogy to the current notion of NR2B interaction with CaMKII, we propose a model that provides a mechanistic framework for CaMKII and Cx36 interaction at electrical synapses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

