The effect of intravenous lidocaine on brain activation during non-noxious and acute noxious stimulation of the forepaw: a functional magnetic resonance imaging study in the rat
- PMID: 19095870
- PMCID: PMC2681082
- DOI: 10.1213/ane.0b013e31818e0d34
The effect of intravenous lidocaine on brain activation during non-noxious and acute noxious stimulation of the forepaw: a functional magnetic resonance imaging study in the rat
Abstract
Background: Lidocaine can alleviate acute as well as chronic neuropathic pain at very low plasma concentrations in humans and laboratory animals. The mechanism(s) underlying lidocaine's analgesic effect when administered systemically is poorly understood but clearly not related to interruption of peripheral nerve conduction. Other targets for lidocaine's analgesic action(s) have been suggested, including sodium channels and other receptor sites in the central rather than peripheral nervous system. To our knowledge, the effect of lidocaine on the brain's functional response to pain has never been investigated. Here, we therefore characterized the effect of systemic lidocaine on the brain's response to innocuous and acute noxious stimulation in the rat using functional magnetic resonance imaging (fMRI).
Methods: Alpha-chloralose anesthetized rats underwent fMRI to quantify brain activation patterns in response to innocuous and noxious forepaw stimulation before and after IV administration of lidocaine.
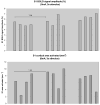
Results: Innocuous forepaw stimulation elicited brain activation only in the contralateral primary somatosensory (S1) cortex. Acute noxious forepaw stimulation induced activation in additional brain areas associated with pain perception, including the secondary somatosensory cortex (S2), thalamus, insula and limbic regions. Lidocaine administered at IV doses of either 1 mg/kg, 4 mg/kg or 10 mg/kg did not abolish or diminish brain activation in response to innocuous or noxious stimulation. In fact, IV doses of 4 mg/kg and 10 mg/kg lidocaine enhanced S1 and S2 responses to acute nociceptive stimulation, increasing the activated cortical volume by 50%-60%.
Conclusion: The analgesic action of systemic lidocaine in acute pain is not reflected in a straightforward interruption of pain-induced fMRI brain activation as has been observed with opioids. The enhancement of cortical fMRI responses to acute pain by lidocaine observed here has also been reported for cocaine. We recently showed that both lidocaine and cocaine increased intracellular calcium concentrations in cortex, suggesting that this pharmacological effect could account for the enhanced sensitivity to somatosensory stimulation. As our model only measured physiological acute pain, it will be important to also test the response of these same pathways to lidocaine in a model of neuropathic pain to further investigate lidocaine's analgesic mechanism of action.
Figures




Similar articles
-
fMRI investigation of the effect of local and systemic lidocaine on noxious electrical stimulation-induced activation in spinal cord.Pain. 2009 Sep;145(1-2):110-9. doi: 10.1016/j.pain.2009.05.026. Epub 2009 Jun 26. Pain. 2009. PMID: 19560271
-
A fMRI study of brain activations during non-noxious and noxious electrical stimulation of the sciatic nerve of rats.Brain Res. 2001 Apr 6;897(1-2):71-81. doi: 10.1016/s0006-8993(01)02094-7. Brain Res. 2001. PMID: 11282360
-
Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation.Neuroimage. 2007 Apr 1;35(2):719-28. doi: 10.1016/j.neuroimage.2006.12.014. Epub 2006 Dec 16. Neuroimage. 2007. PMID: 17300960
-
Functional imaging of brain responses to pain. A review and meta-analysis (2000).Neurophysiol Clin. 2000 Oct;30(5):263-88. doi: 10.1016/s0987-7053(00)00227-6. Neurophysiol Clin. 2000. PMID: 11126640 Review.
-
Population Pharmacokinetics of Intravenous Lidocaine in Adults: A Systematic Review.Clin Pharmacokinet. 2024 May;63(5):623-643. doi: 10.1007/s40262-024-01373-4. Epub 2024 May 4. Clin Pharmacokinet. 2024. PMID: 38703307
Cited by
-
Intravenous administration of lidocaine directly acts on spinal dorsal horn and produces analgesic effect: An in vivo patch-clamp analysis.Sci Rep. 2016 May 18;6:26253. doi: 10.1038/srep26253. Sci Rep. 2016. PMID: 27188335 Free PMC article.
-
Intrathecal lidocaine pretreatment attenuates immediate neuropathic pain by modulating Nav1.3 expression and decreasing spinal microglial activation.BMC Neurol. 2011 Jun 16;11:71. doi: 10.1186/1471-2377-11-71. BMC Neurol. 2011. PMID: 21676267 Free PMC article.
-
New index of pain triggered by spinal activation of voltage-dependent sodium channels.J Anesth. 2013 Dec;27(6):939-41. doi: 10.1007/s00540-013-1646-0. Epub 2013 Jun 13. J Anesth. 2013. PMID: 23760511
-
Lidocaine patch (5%) is no more potent than placebo in treating chronic back pain when tested in a randomised double blind placebo controlled brain imaging study.Mol Pain. 2012 Apr 24;8:29. doi: 10.1186/1744-8069-8-29. Mol Pain. 2012. PMID: 22531485 Free PMC article. Clinical Trial.
-
The biological effect of contralateral forepaw stimulation in rat focal cerebral ischemia: a multispectral optical imaging study.Front Neuroenergetics. 2010 Jul 30;2:19. doi: 10.3389/fnene.2010.00019. eCollection 2010. Front Neuroenergetics. 2010. PMID: 20725601 Free PMC article.
References
-
- Bonica J. The management of pain. Philadelphia: Lea & Febiger; 1953.
-
- Cassuto J, Wallin G, Hogstrom S, Faxen A, Rimback G. Inhibition of postoperative pain by continuous low-dose intravenous infusion of lidocaine. Anesth Analg. 1985;64:971–4. - PubMed
-
- Groudine SB, Fisher HA, Kaufman RP, Jr, Patel MK, Wilkins LJ, Mehta SA, Lumb PD. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg. 1998;86:235–9. - PubMed
-
- Matharu MS, Cohen AS, Goadsby PJ. SUNCT syndrome responsive to intravenous lidocaine. Cephalalgia. 2004;24:985–92. - PubMed
-
- Jonsson A, Cassuto J, Hanson B. Inhibition of burn pain by intravenous lignocaine infusion. Lancet. 1991;338:151–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

