Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2
- PMID: 19096031
- PMCID: PMC2720065
- DOI: 10.1161/CIRCRESAHA.108.181040
Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2
Abstract
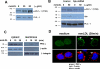
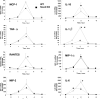
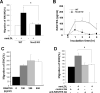
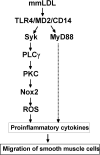
Oxidative modification of low-density lipoprotein (LDL) plays a causative role in the development of atherosclerosis. In this study, we demonstrate that minimally oxidized LDL (mmLDL) stimulates intracellular reactive oxygen species (ROS) generation in macrophages through NADPH oxidase 2 (gp91phox/Nox2), which, in turn, induces production of RANTES and migration of smooth muscle cells. Peritoneal macrophages from gp91phox/Nox2(-/-) mice or J774 macrophages in which Nox2 was knocked down by small interfering RNA failed to generate ROS in response to mmLDL. Because mmLDL-induced cytoskeletal changes were dependent on Toll-like receptor (TLR)4, we analyzed ROS generation in peritoneal macrophages from wild-type, TLR4(-/-), or MyD88(-/-) mice and found that mmLDL-mediated ROS was generated in a TLR4-dependent, but MyD88-independent, manner. Furthermore, we found that ROS generation required the recruitment and activation of spleen tyrosine kinase (Syk) and that mmLDL also induced phospholipase PLCgamma1 phosphorylation and protein kinase C membrane translocation. Importantly, the phospholipase Cgamma1 phosphorylation was reduced in J774 cells expressing Syk-specific short hairpin RNA. Nox2 modulated mmLDL activation of macrophages by regulating the expression of proinflammatory cytokines interleukin-1beta, interleukin-6, and RANTES. We showed that purified RANTES was able to stimulate migration of mouse aortic smooth muscle cells and addition of neutralizing antibody against RANTES abolished the migration of mouse aortic smooth muscle cells stimulated by mmLDL-stimulated macrophages. These results suggest that mmLDL induces generation of ROS through sequential activation of TLR4, Syk, phospholipase Cgamma1, protein kinase C, and gp91phox/Nox2 and thereby stimulates expression of proinflammatory cytokines. These data help explain mechanisms by which endogenous ligands, such as mmLDL, can induce TLR4-dependent, proatherogenic activation of macrophages.
Figures





References
-
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. - PubMed
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
-
- Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. - PubMed
-
- Kawai T, Akira S. TLR signaling. Seminars in Immunol. 2007;19:24–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

