Heparanase alters arterial structure, mechanics, and repair following endovascular stenting in mice
- PMID: 19096032
- PMCID: PMC2805167
- DOI: 10.1161/CIRCRESAHA.108.180695
Heparanase alters arterial structure, mechanics, and repair following endovascular stenting in mice
Abstract
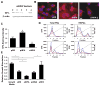
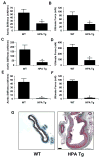
Heparan sulfate proteoglycans (HSPGs) are potent regulators of vascular remodeling and repair. Heparanase is the major enzyme capable of degrading heparan sulfate in mammalian cells. Here we examined the role of heparanase in controlling arterial structure, mechanics, and remodeling. In vitro studies supported that heparanase expression in endothelial cells serves as a negative regulator of endothelial inhibition of vascular smooth muscle cell (vSMC) proliferation. Arterial structure and remodeling to injury were also modified by heparanase expression. Transgenic mice overexpressing heparanase had increased arterial thickness, cellular density, and mechanical compliance. Endovascular stenting studies in Zucker rats demonstrated increased heparanase expression in the neointima of obese, hyperlipidemic rats in comparison to lean rats. The extent of heparanase expression within the neointima strongly correlated with the neointimal thickness following injury. To test the effects of heparanase overexpression on arterial repair, we developed a novel murine model of stent injury using small diameter self-expanding stents. Using this model, we found that increased neointimal formation and macrophage recruitment occurs in transgenic mice overexpressing heparanase. Taken together, these results support a role for heparanase in the regulation of arterial structure, mechanics, and repair.
Figures





Comment in
-
Getting neointimal: the emergence of heparanase into the vascular matrix.Circ Res. 2009 Feb 13;104(3):277-9. doi: 10.1161/CIRCRESAHA.108.192625. Circ Res. 2009. PMID: 19213960 No abstract available.
References
-
- Libby P. Atherosclerosis: disease biology affecting the coronary vasculature. Am J Cardiol. 2006;98:3Q–9Q. - PubMed
-
- Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007;100:3K–9K. - PubMed
-
- Karnovsky MJ, Edelman ER. Heparin/heparan sulphate regulation of vascular smooth muscle behaviour. In: Page CP, Black JL, editors. Airways and Vascular Remodeling in Asthma and Cardiovascular Disease: Implications for Therapeutic Intervention. New York: Academic Press; 1994. pp. 45–70.
-
- Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196:430–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

