Improved Insulin Resistance and Lipid Metabolism by Cinnamon Extract through Activation of Peroxisome Proliferator-Activated Receptors
- PMID: 19096709
- PMCID: PMC2602825
- DOI: 10.1155/2008/581348
Improved Insulin Resistance and Lipid Metabolism by Cinnamon Extract through Activation of Peroxisome Proliferator-Activated Receptors
Abstract
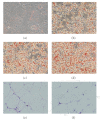
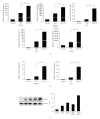
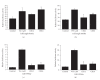
Peroxisome proliferator-activated receptors (PPARs) are transcriptional factors involved in the regulation of insulin resistance and adipogenesis. Cinnamon, a widely used spice in food preparation and traditional antidiabetic remedy, is found to activate PPARgamma and alpha, resulting in improved insulin resistance, reduced fasted glucose, FFA, LDL-c, and AST levels in high-caloric diet-induced obesity (DIO) and db/db mice in its water extract form. In vitro studies demonstrate that cinnamon increases the expression of peroxisome proliferator-activated receptors gamma and alpha (PPARgamma/alpha) and their target genes such as LPL, CD36, GLUT4, and ACO in 3T3-L1 adipocyte. The transactivities of both full length and ligand-binding domain (LBD) of PPARgamma and PPARalpha are activated by cinnamon as evidenced by reporter gene assays. These data suggest that cinnamon in its water extract form can act as a dual activator of PPARgamma and alpha, and may be an alternative to PPARgamma activator in managing obesity-related diabetes and hyperlipidemia.
Figures

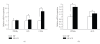
References
-
- DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. - PubMed
-
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine Reviews. 1999;20(5):649–688. - PubMed
-
- Steiner G, Hamsten A, Hosking J, et al. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. The Lancet. 2001;357(9260):905–910. - PubMed
-
- Cleeman JI. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) The Journal of the American Medical Association. 2001;285(19):2486–2497. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

