Steady state bioequivalence of generic and innovator formulations of stavudine, lamivudine, and nevirapine in HIV-infected Ugandan adults
- PMID: 19096711
- PMCID: PMC2602850
- DOI: 10.1371/journal.pone.0003981
Steady state bioequivalence of generic and innovator formulations of stavudine, lamivudine, and nevirapine in HIV-infected Ugandan adults
Abstract
Background: Generic antiretroviral therapy is the mainstay of HIV treatment in resource-limited settings, yet there is little evidence confirming the bioequivalence of generic and brand name formulations. We compared the steady-state pharmacokinetics of lamivudine, stavudine and nevirapine in HIV-infected subjects who were receiving a generic formulation (Triomune) or the corresponding brand formulations (Epivir, Zerit, and Viramune).
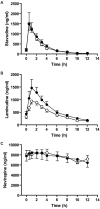
Methodology/principal findings: An open-label, randomized, crossover study was carried out in 18 HIV-infected Ugandan subjects stabilized on Triomune-40. Subjects received lamivudine (150 mg), stavudine (40 mg), and nevirapine (200 mg) in either the generic or brand formulation twice a day for 30 days, before switching to the other formulation. At the end of each treatment period, blood samples were collected over 12 h for pharmacokinetic analysis. The main outcome measures were the mean AUC(0-12h) and C(max). Bioequivalence was defined as a geometric mean ratio between the generic and brand name within the 90% confidence interval of 0.8-1.25. The geometric mean ratios and the 90% confidence intervals were: stavudine C(max), 1.3 (0.99-1.71) and AUC(0-12h), 1.1 (0.87-1.38); lamivudine C(max), 0.8 (0.63-0.98) and AUC(0-12h), 0.8 (0.65-0.99); and nevirapine C(max), 1.1 (0.95-1.23) and AUC(0-12h), 1.1 (0.95-1.31). The generic formulation was not statistically bioequivalent to the brand formulations during steady state, although exposures were comparable. A mixed random effects model identified about 50% intersubject variability in the pharmacokinetic parameters.
Conclusions/significant findings: These findings provide support for the use of Triomune in resource-limited settings, although identification of the sources of intersubject variability in these populations is critical.
Conflict of interest statement
Figures
References
-
- Narang VS, Lulla A, Malhotra G, Purandare S. A combined-formulation tablet of lamivudine/nevirapine/stavudine: bioequivalence compared with concurrent administration of lamivudine, nevirapine, and stavudine in healthy Indian subjects. J Clin Pharmacol. 2005;45:265–274. - PubMed
-
- Hosseinipour MC, Corbett AH, Kanyama C, Mshali I, Phakati S, et al. Pharmacokinetic comparison of generic and trade formulations of lamivudine, stavudine and nevirapine in HIV-infected Malawian adults. AIDS. 2007;21:59–64. - PubMed
-
- Oyugi JH, Byakika-Tusiime J, Charlebois ED, Kityo C, Mugerwa R, et al. Multiple validated measures of adherence indicate high levels of adherence to generic HIV antiretroviral therapy in a resource-limited setting. J Acquir Immune Defic Syndr. 2004;36:1100–1102. - PubMed
-
- Oyugi JH, Byakika-Tusiime J, Ragland K, Laeyendecker O, Mugerwa R, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21:965–971. - PubMed
-
- Mistri HN, Jangid AG, Pudage A, Gomes N, Sanyal M, et al. High throughput LC-MS/MS method for simultaneous quantification of lamivudine, stavudine and nevirapine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853:320–332. - PubMed

