High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography
- PMID: 19096730
- PMCID: PMC2605071
- DOI: 10.1364/OE.14.004380
High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography
Abstract
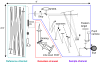
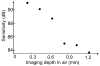
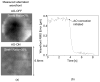
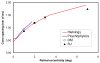
We report the first observations of the three-dimensional morphology of cone photoreceptors in the living human retina. Images were acquired with a high-speed adaptive optics (AO) spectral-domain optical coherence tomography (SD-OCT) camera. The AO system consisted of a Shack-Hartmann wavefront sensor and bimorph mirror (AOptix) that measured and corrected the ocular and system aberrations at a closed-loop rate of 12 Hz. The bimorph mirror was positioned between the XY mechanical scanners and the subject's eye. The SD-OCT system consisted of a superluminescent diode and a 512 pixel line scan charge-coupled device (CCD) that acquired 75,000 A-scans/s. This rate is more than two times faster than that previously reported. Retinal motion artifacts were minimized by quickly acquiring small volume images of the retina with and without AO compensation. Camera sensitivity was sufficient to detect reflections from all major retinal layers. The regular distribution of bright spots observed within C-scans at the inner segment / outer segment (IS/OS) junctions and at the posterior tips of the OS were found to be highly correlated with one another and with the expected cone spacing. No correlation was found between the posterior tips of the OS and the other retinal layers examined, including the retinal pigment epithelium.
Figures






References
-
- Fercher AF, Hitzenberger CK, Kamp G, Elzaiat Y. Measurement of intraocular distance by backscattering spectral interferometry. Opt Commun. 1995;117:43–48.
-
- Wojtkowski M, Leitgeb RA, Kowalczyk A, Bajraszewski T, Fercher AF. In vivo human retinal imaging by Fourier domain optical coherence tomography. J Biomed Opt. 2002;7:457–463. - PubMed
-
- Cense B, Nassif NA, Chen TC, Pierce MC, Yun S, Park BH, Bouma BE, Tearney GJ, de Boer JF. Ultrahigh-resolution high-speed retinal imaging using spectral-domain optical coherence tomography. Opt Express. 2004;12:2435–2447. - PubMed
-
- Wojtkowski M, Srinivasan VJ, Ko TH, Fujimoto JG, Kowalczyk A, Duker JS. Ultrahigh-resolution, high-speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Opt Express. 2004;12:2404–2421. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

