Transcriptional dynamics of endodermal organ formation
- PMID: 19097184
- PMCID: PMC3756511
- DOI: 10.1002/dvdy.21810
Transcriptional dynamics of endodermal organ formation
Abstract
Although endodermal organs including the liver, pancreas, and intestine are of significant therapeutic interest, the mechanism by which the endoderm is divided into organ domains during embryogenesis is not well understood. To better understand this process, global gene expression profiling was performed on early endodermal organ domains. This global analysis was followed up by dynamic immunofluorescence analysis of key transcription factors, uncovering novel expression patterns as well as cell surface proteins that allow prospective isolation of specific endodermal organ domains. Additionally, a repressive interaction between Cdx2 and Sox2 was found to occur at the prospective stomach-intestine border, with the hepatic and pancreatic domains forming at this boundary, and Hlxb9 was revealed to have graded expression along the dorsal-ventral axis. These results contribute to understanding the mechanism of endodermal organogenesis and should assist efforts to replicate this process using pluripotent stem cells.
Copyright (c) 2008 Wiley-Liss, Inc.
Figures

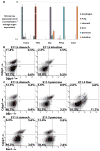


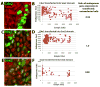


References
-
- Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–1315. - PubMed
-
- Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–227. - PubMed
-
- Beck F, Tata F, Chawengsaksophak K. Homeobox genes and gut development. Bioessays. 2000;22:431–441. - PubMed
-
- Burke Z, Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech Dev. 2002;118:147–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

