Stereo-controlled synthesis of novel photoreactive gamma-secretase inhibitors
- PMID: 19097779
- PMCID: PMC3254698
- DOI: 10.1016/j.bmcl.2008.11.117
Stereo-controlled synthesis of novel photoreactive gamma-secretase inhibitors
Abstract
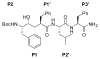
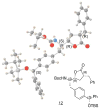
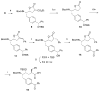
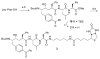
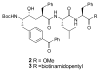
The stereoselective synthesis of novel photoreactive gamma-secretase inhibitors 2 and 3 has been achieved. Key steps of the strategy involve preparation of alpha-N-Boc-epoxide 8 and formation of lactone 14 in a practical and stereo-controlled fashion. Compounds 2 and 3 are potent gamma-secretase inhibitors and directly interact with presenilin-1, a catalytic subunit of gamma-secretase.
Figures

References
-
- Hardy J, Allsop D. Trends Pharmacol Sci. 1991;12:383. - PubMed
-
- De Strooper B. Neuron. 2003;38:9. - PubMed
-
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Nature. 1999;398:513. - PubMed
-
- Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ. Nature. 2000;405:689. - PubMed
-
- Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai JY, Rahmati T, Xia W, Selkoe DJ, Wolfe MS. Nat Cell Biol. 2000;2:428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

