Purinergic P2X7 receptors mediate ATP-induced saliva secretion by the mouse submandibular gland
- PMID: 19097994
- PMCID: PMC2643514
- DOI: 10.1074/jbc.M808597200
Purinergic P2X7 receptors mediate ATP-induced saliva secretion by the mouse submandibular gland
Abstract
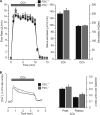
Salivary glands express multiple isoforms of P2X and P2Y nucleotide receptors, but their in vivo physiological roles are unclear. P2 receptor agonists induced salivation in an ex vivo submandibular gland preparation. The nucleotide selectivity sequence of the secretion response was BzATP >> ATP > ADP >> UTP, and removal of external Ca(2+) dramatically suppressed the initial ATP-induced fluid secretion ( approximately 85%). Together, these results suggested that P2X receptors are the major purinergic receptor subfamily involved in the fluid secretion process. Mice with targeted disruption of the P2X(7) gene were used to evaluate the role of the P2X(7) receptor in nucleotide-evoked fluid secretion. P2X(7) receptor protein and BzATP-activated inward cation currents were absent, and importantly, purinergic receptor agonist-stimulated salivation was suppressed by more than 70% in submandibular glands from P2X(7)-null mice. Consistent with these observations, the ATP-induced increases in [Ca(2+)](i) were nearly abolished in P2X(7)(-/-) submandibular acinar and duct cells. ATP appeared to also act through the P2X(7) receptor to inhibit muscarinic-induced fluid secretion. These results demonstrate that the ATP-sensitive P2X(7) receptor regulates fluid secretion in the mouse submandibular gland.
Figures
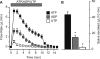





Similar articles
-
Contribution of two ionotropic purinergic receptors to ATP responses in submandibular gland ductal cells.Cell Signal. 2007 Oct;19(10):2155-64. doi: 10.1016/j.cellsig.2007.06.012. Epub 2007 Jun 28. Cell Signal. 2007. PMID: 17651941
-
Stimulation by P2X₇ receptors of calcium-dependent production of reactive oxygen species (ROS) in rat submandibular glands.Biochim Biophys Acta. 2010 Nov;1800(11):1183-91. doi: 10.1016/j.bbagen.2010.07.007. Epub 2010 Jul 23. Biochim Biophys Acta. 2010. PMID: 20655985
-
Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl(-) reabsorption in rat submandibular gland.Am J Physiol Regul Integr Comp Physiol. 2008 May;294(5):R1729-36. doi: 10.1152/ajpregu.00758.2007. Epub 2008 Mar 12. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18337312
-
ATP ion channel P2X purinergic receptors in inflammation response.Biomed Pharmacother. 2023 Feb;158:114205. doi: 10.1016/j.biopha.2022.114205. Epub 2023 Jan 4. Biomed Pharmacother. 2023. PMID: 36916431 Review.
-
Roles of P2X7 receptor in glial and neuroblastoma cells: the therapeutic potential of P2X7 receptor antagonists.Mol Neurobiol. 2010 Jun;41(2-3):351-5. doi: 10.1007/s12035-010-8120-x. Epub 2010 Apr 21. Mol Neurobiol. 2010. PMID: 20405342 Review.
Cited by
-
Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion.Physiol Rev. 2012 Jan;92(1):39-74. doi: 10.1152/physrev.00011.2011. Physiol Rev. 2012. PMID: 22298651 Free PMC article. Review.
-
Advances in cellular and molecular pathways of salivary gland damage in Sjögren's syndrome.Front Immunol. 2024 Jul 10;15:1405126. doi: 10.3389/fimmu.2024.1405126. eCollection 2024. Front Immunol. 2024. PMID: 39050857 Free PMC article. Review.
-
P2X(7) receptor antagonists display agonist-like effects on cell signaling proteins.Biochim Biophys Acta. 2011 May;1810(5):532-42. doi: 10.1016/j.bbagen.2011.03.009. Epub 2011 Mar 21. Biochim Biophys Acta. 2011. PMID: 21397667 Free PMC article.
-
Activation of ERK1/2 by store-operated calcium entry in rat parotid acinar cells.PLoS One. 2013 Aug 29;8(8):e72881. doi: 10.1371/journal.pone.0072881. eCollection 2013. PLoS One. 2013. PMID: 24009711 Free PMC article.
-
Duct ligation/de-ligation model: exploring mechanisms for salivary gland injury and regeneration.Front Cell Dev Biol. 2024 Jun 25;12:1399934. doi: 10.3389/fcell.2024.1399934. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38983787 Free PMC article. Review.
References
-
- Cook, D. I., Van Lennep, E. W., Roberts, M. L., and Young, J. A. (1994) Secretion by the Major Salivary Glands, 3rd Ed., Raven Press, New York
-
- Melvin, J. E., Yule, D., Shuttleworth, T., and Begenisich, T. (2005) Annu. Rev. Physiol. 67 445–469 - PubMed
-
- Ekstrom, J., Mansson, B., Olgart, L., and Tobin, G. (1988) Quart. J. Exp. Physiol. 73 163–173 - PubMed
-
- Merritt, J. E., and Rink, T. J. (1987) J. Biol. Chem. 262 14912–14916 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

