Humanized Mouse Model of Cooley's Anemia
- PMID: 19098001
- PMCID: PMC2643510
- DOI: 10.1074/jbc.M805681200
Humanized Mouse Model of Cooley's Anemia
Abstract
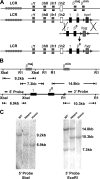
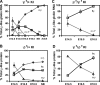
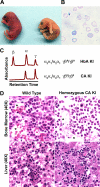
A novel humanized mouse model of Cooley's Anemia (CA) was generated by targeted gene replacement in embryonic stem (ES) cells. Because the mouse does not have a true fetal hemoglobin, a delayed switching human gamma to beta(0) globin gene cassette (gammabeta(0)) was inserted directly into the murine beta globin locus replacing both adult mouse beta globin genes. The inserted human beta(0) globin allele has a mutation in the splice donor site that produces the same aberrant transcripts in mice as described in human cells. No functional human beta globin polypeptide chains are produced. Heterozygous gammabeta(0) mice suffer from microcytic anemia. Unlike previously described animal models of beta thalassemia major, homozygous gammabeta(0) mice switch from mouse embryonic globin chains to human fetal gamma globin during fetal life. When bred with human alpha globin knockin mice, homozygous CA mice survive solely upon human fetal hemoglobin at birth. This preclinical animal model of CA can be utilized to study the regulation of globin gene expression, synthesis, and switching; the reactivation of human fetal globin gene expression; and the testing of genetic and cell-based therapies for the correction of thalassemia.
Figures


Similar articles
-
Humanized mouse models of Cooley's anemia: correct fetal-to-adult hemoglobin switching, disease onset, and disease pathology.Ann N Y Acad Sci. 2010 Aug;1202:45-51. doi: 10.1111/j.1749-6632.2010.05547.x. Ann N Y Acad Sci. 2010. PMID: 20712771 Free PMC article.
-
Preclinical transfusion-dependent humanized mouse model of beta thalassemia major.Blood. 2009 May 7;113(19):4763-70. doi: 10.1182/blood-2008-12-197012. Epub 2009 Mar 3. Blood. 2009. PMID: 19258591 Free PMC article.
-
Non-anemic homozygous beta(o) thalassemia in an African-American family: association of high fetal hemoglobin levels with beta thalassemia alleles.Am J Hematol. 2001 Sep;68(1):43-50. doi: 10.1002/ajh.1147. Am J Hematol. 2001. PMID: 11559936
-
Role of intergenic human gamma-delta-globin sequences in human hemoglobin switching and reactivation of fetal hemoglobin in adult erythroid cells.Ann N Y Acad Sci. 2005;1054:48-54. doi: 10.1196/annals.1345.057. Ann N Y Acad Sci. 2005. PMID: 16339651 Review.
-
Targeted fetal hemoglobin induction for treatment of beta hemoglobinopathies.Hematol Oncol Clin North Am. 2014 Apr;28(2):233-48. doi: 10.1016/j.hoc.2013.11.009. Hematol Oncol Clin North Am. 2014. PMID: 24589264 Review.
Cited by
-
In Utero Gene Therapy (IUGT) Using GLOBE Lentiviral Vector Phenotypically Corrects the Heterozygous Humanised Mouse Model and Its Progress Can Be Monitored Using MRI Techniques.Sci Rep. 2019 Aug 12;9(1):11592. doi: 10.1038/s41598-019-48078-4. Sci Rep. 2019. PMID: 31406195 Free PMC article.
-
Animal Models of Normal and Disturbed Iron and Copper Metabolism.J Nutr. 2019 Dec 1;149(12):2085-2100. doi: 10.1093/jn/nxz172. J Nutr. 2019. PMID: 31504675 Free PMC article. Review.
-
Non-invasive MRI biomarkers for the early assessment of iron overload in a humanized mouse model of β-thalassemia.Sci Rep. 2017 Feb 27;7:43439. doi: 10.1038/srep43439. Sci Rep. 2017. PMID: 28240317 Free PMC article.
-
Minihepcidins improve ineffective erythropoiesis and splenomegaly in a new mouse model of adult β-thalassemia major.Haematologica. 2020 Jul;105(7):1835-1844. doi: 10.3324/haematol.2018.212589. Epub 2019 Oct 3. Haematologica. 2020. PMID: 31582543 Free PMC article.
-
Erythropoiesis in the absence of adult hemoglobin.Mol Cell Biol. 2013 Jun;33(11):2241-51. doi: 10.1128/MCB.01734-12. Epub 2013 Mar 25. Mol Cell Biol. 2013. PMID: 23530053 Free PMC article.
References
-
- Bank, A. (1985) Curr. Top. Hematol. 5 1–23 - PubMed
-
- Bunn, H., and Forget, B. (1986) Hemoglobin: Molecular, Genetic, and Clinical Aspects, pp. 333–343, W. B. Saunders, Philadelphia
-
- Steinberg, M. H. (1988) Am. J. Med. Sci. 296 308–321 - PubMed
-
- Mathias, L. A., Fisher, T. C., Zeng, L., Meiselman, H. J., Weinberg, K. I., Hiti, A. L., and Malik, P. (2000) Exp. Hematol. 28 1343–1353 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

