Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots
- PMID: 19098091
- PMCID: PMC2633862
- DOI: 10.1104/pp.108.132811
Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots
Abstract
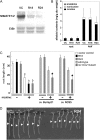
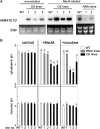
Nicotine is a major alkaloid accumulating in the vacuole of tobacco (Nicotiana tabacum), but the transporters involved in the vacuolar sequestration are not known. We here report that tobacco genes (NtMATE1 and NtMATE2) encoding transporters of the multidrug and toxic compound extrusion (MATE) family are coordinately regulated with structural genes for nicotine biosynthesis in the root, with respect to spatial expression patterns, regulation by NIC regulatory loci, and induction by methyl jasmonate. Subcellular fractionation, immunogold electron microscopy, and expression of a green fluorescent protein fusion protein all suggested that these transporters are localized to the vacuolar membrane. Reduced expression of the transporters rendered tobacco plants more sensitive to the application of nicotine. In contrast, overexpression of NtMATE1 in cultured tobacco cells induced strong acidification of the cytoplasm after jasmonate elicitation or after the addition of nicotine under nonelicited conditions. Expression of NtMATE1 in yeast (Saccharomyces cerevisiae) cells compromised the accumulation of exogenously supplied nicotine into the yeast cells. The results imply that these MATE-type proteins transport tobacco alkaloids from the cytosol into the vacuole in exchange for protons in alkaloid-synthesizing root cells.
Figures




References
-
- Balandin T, Vanderdoes C, Albert JMB, Bol JF, Linthorst HJM (1995) Structure and induction pattern of a novel proteinase-inhibitor class-II gene of tobacco. Plant Mol Biol 27 1197–1204 - PubMed
-
- Baldwin IT (1989) Mechanism of damage-induced alkaloid production in wild tobacco. J Chem Ecol 15 1661–1680 - PubMed
-
- Blingny R, Douce R (2001) NMR and plant metabolism. Curr Opin Plant Biol 4 191–196 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

