Regulation of respiration and fermentation to control the plant internal oxygen concentration
- PMID: 19098094
- PMCID: PMC2633817
- DOI: 10.1104/pp.108.129288
Regulation of respiration and fermentation to control the plant internal oxygen concentration
Abstract
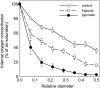
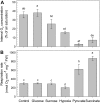
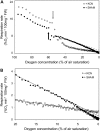
Plant internal oxygen concentrations can drop well below ambient even when the plant grows under optimal conditions. Using pea (Pisum sativum) roots, we show how amenable respiration adapts to hypoxia to save oxygen when the oxygen availability decreases. The data cannot simply be explained by oxygen being limiting as substrate but indicate the existence of a regulatory mechanism, because the oxygen concentration at which the adaptive response is initiated is independent of the actual respiratory rate. Two phases can be discerned during the adaptive reaction: an initial linear decline of respiration is followed by a nonlinear inhibition in which the respiratory rate decreased progressively faster upon decreasing oxygen availability. In contrast to the cytochrome c pathway, the inhibition of the alternative oxidase pathway shows only the linear component of the adaptive response. Feeding pyruvate to the roots led to an increase of the oxygen consumption rate, which ultimately led to anoxia. The importance of balancing the in vivo pyruvate availability in the tissue was further investigated. Using various alcohol dehydrogenase knockout lines of Arabidopsis (Arabidopsis thaliana), it was shown that even under aerobic conditions, alcohol fermentation plays an important role in the control of the level of pyruvate in the tissue. Interestingly, alcohol fermentation appeared to be primarily induced by a drop in the energy status of the tissue rather than by a low oxygen concentration, indicating that sensing the energy status is an important component of optimizing plant metabolism to changes in the oxygen availability.
Figures
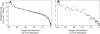


References
-
- Armstrong W, Strange ME, Cringle S, Beckett PM (1994) Microelectrode and modelling study of oxygen distribution in roots. Ann Bot (Lond) 74 287–299
-
- Banti V, Loreti E, Novi G, Santaniello A, Alpi A, Perata P (2008) Heat acclimation and cross-tolerance against anoxia in Arabidopsis. Plant Cell Environ 31 1029–1037 - PubMed
-
- Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59 313–339 - PubMed
-
- Benson BB, Krause D (1980) The concentration and isotopic fractionation of gases dissolved in freshwater in equilibrium with the atmosphere. 1. Oxygen. Limnol Oceanogr 25 662–671
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

