Adaptive protein evolution grants organismal fitness by improving catalysis and flexibility
- PMID: 19098096
- PMCID: PMC2634896
- DOI: 10.1073/pnas.0807989106
Adaptive protein evolution grants organismal fitness by improving catalysis and flexibility
Abstract
Protein evolution is crucial for organismal adaptation and fitness. This process takes place by shaping a given 3-dimensional fold for its particular biochemical function within the metabolic requirements and constraints of the environment. The complex interplay between sequence, structure, functionality, and stability that gives rise to a particular phenotype has limited the identification of traits acquired through evolution. This is further complicated by the fact that mutations are pleiotropic, and interactions between mutations are not always understood. Antibiotic resistance mediated by beta-lactamases represents an evolutionary paradigm in which organismal fitness depends on the catalytic efficiency of a single enzyme. Based on this, we have dissected the structural and mechanistic features acquired by an optimized metallo-beta-lactamase (MbetaL) obtained by directed evolution. We show that antibiotic resistance mediated by this enzyme is driven by 2 mutations with sign epistasis. One mutation stabilizes a catalytically relevant intermediate by fine tuning the position of 1 metal ion; whereas the other acts by augmenting the protein flexibility. We found that enzyme evolution (and the associated antibiotic resistance) occurred at the expense of the protein stability, revealing that MbetaLs have not exhausted their stability threshold. Our results demonstrate that flexibility is an essential trait that can be acquired during evolution on stable protein scaffolds. Directed evolution aided by a thorough characterization of the selected proteins can be successfully used to predict future evolutionary events and design inhibitors with an evolutionary perspective.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
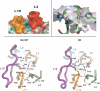
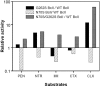

Similar articles
-
Mimicking natural evolution in metallo-beta-lactamases through second-shell ligand mutations.Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):13761-6. doi: 10.1073/pnas.0503495102. Epub 2005 Sep 19. Proc Natl Acad Sci U S A. 2005. PMID: 16172409 Free PMC article.
-
Optimization of Conformational Dynamics in an Epistatic Evolutionary Trajectory.Mol Biol Evol. 2016 Jul;33(7):1768-76. doi: 10.1093/molbev/msw052. Epub 2016 Mar 15. Mol Biol Evol. 2016. PMID: 26983555 Free PMC article.
-
Evidence for a dinuclear active site in the metallo-beta-lactamase BcII with substoichiometric Co(II). A new model for metal uptake.J Biol Chem. 2007 Oct 19;282(42):30586-95. doi: 10.1074/jbc.M704613200. Epub 2007 Aug 22. J Biol Chem. 2007. PMID: 17715135
-
Metallo-β-Lactamases: Influence of the Active Site Structure on the Mechanisms of Antibiotic Resistance and Inhibition.Biochemistry (Mosc). 2021 Jan;86(Suppl 1):S24-S37. doi: 10.1134/S0006297921140030. Biochemistry (Mosc). 2021. PMID: 33827398 Review.
-
Structure, Function of Serine and Metallo-β-lactamases and their Inhibitors.Curr Protein Pept Sci. 2018;19(2):130-144. doi: 10.2174/0929866524666170724160623. Curr Protein Pept Sci. 2018. PMID: 28745223 Review.
Cited by
-
Molecular Evolution of Transition Metal Bioavailability at the Host-Pathogen Interface.Trends Microbiol. 2021 May;29(5):441-457. doi: 10.1016/j.tim.2020.08.001. Epub 2020 Sep 18. Trends Microbiol. 2021. PMID: 32951986 Free PMC article. Review.
-
Evolution of Metallo-β-lactamases: Trends Revealed by Natural Diversity and in vitro Evolution.Antibiotics (Basel). 2014 Jul 1;3(3):285-316. doi: 10.3390/antibiotics3030285. Antibiotics (Basel). 2014. PMID: 25364574 Free PMC article.
-
Dual Activity BLEG-1 from Bacillus lehensis G1 Revealed Structural Resemblance to B3 Metallo-β-Lactamase and Glyoxalase II: An Insight into Its Enzyme Promiscuity and Evolutionary Divergence.Int J Mol Sci. 2021 Aug 29;22(17):9377. doi: 10.3390/ijms22179377. Int J Mol Sci. 2021. PMID: 34502284 Free PMC article.
-
Overcoming differences: The catalytic mechanism of metallo-β-lactamases.FEBS Lett. 2015 Nov 14;589(22):3419-32. doi: 10.1016/j.febslet.2015.08.015. Epub 2015 Aug 20. FEBS Lett. 2015. PMID: 26297824 Free PMC article. Review.
-
Frustration can Limit the Adaptation of Promiscuous Enzymes Through Gene Duplication and Specialisation.J Mol Evol. 2024 Apr;92(2):104-120. doi: 10.1007/s00239-024-10161-4. Epub 2024 Mar 12. J Mol Evol. 2024. PMID: 38470504 Free PMC article.
References
-
- Poelwijk FJ, Kiviet DJ, Weinreich DM, Tans SJ. Empirical fitness landscapes reveal accessible evolutionary paths. Nature. 2007;445:383–386. - PubMed
-
- Depristo MA, Weinreich DM, Hartl DL. Missense meanderings in sequence space: A biophysical view of protein evolution. Nat Rev Genet. 2005;6:678–687. - PubMed
-
- Bershtein S, Segal M, Bekerman R, Tokuriki N, Tawfik DS. Robustness-epistasis link shapes the fitness landscape of a randomly drifting protein. Nature. 2006;444:929–932. - PubMed
-
- Weinreich DM, Delaney NF, Depristo MA, Hartl DL. Darwinian evolution can follow only very few mutational paths to fitter proteins. Science. 2006;312:111–114. - PubMed
-
- Orencia MC, Yoon JS, Ness JE, Stemmer WP, Stevens RC. Predicting the emergence of antibiotic resistance by directed evolution and structural analysis. Nat Struct Biol. 2001;8:238–242. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

