Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase
- PMID: 19098100
- PMCID: PMC2634948
- DOI: 10.1073/pnas.0808953105
Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase
Abstract
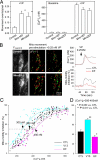
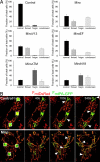
Calcium oscillations suppress mitochondrial movements along the microtubules to support on-demand distribution of mitochondria. To activate this mechanism, Ca(2+) targets a yet unidentified cytoplasmic factor that does not seem to be a microtubular motor or a kinase/phosphatase. Here, we have studied the dependence of mitochondrial dynamics on the Miro GTPases that reside in the mitochondria and contain two EF-hand Ca(2+)-binding domains, in H9c2 cells and primary neurons. At resting cytoplasmic [Ca(2+)] ([Ca(2+)](c)), movements of the mitochondria were enhanced by Miro overexpression irrespective of the presence of the EF-hands. The Ca(2+)-induced arrest of mitochondrial motility was also promoted by Miro overexpression and was suppressed when either the Miro were depleted or their EF-hand was mutated. Miro also enhanced the fusion state of the mitochondria at resting [Ca(2+)](c) but promoted mitochondrial fragmentation at high [Ca(2+)](c). These effects of Miro on mitochondrial morphology seem to involve Drp1 suppression and activation, respectively. In primary neurons, Miro also caused an increase in dendritic mitochondrial mass and enhanced mitochondrial calcium signaling. Thus, Miro proteins serve as a [Ca(2+)](c)-sensitive switch and bifunctional regulator for both the motility and fusion-fission dynamics of the mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

