Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels
- PMID: 19098106
- PMCID: PMC2605631
- DOI: 10.1073/pnas.0806700106
Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels
Abstract
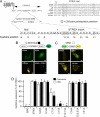
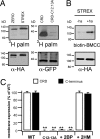
Large conductance calcium- and voltage-gated potassium (BK) channels are important regulators of physiological homeostasis and their function is potently modulated by protein kinase A (PKA) phosphorylation. PKA regulates the channel through phosphorylation of residues within the intracellular C terminus of the pore-forming alpha-subunits. However, the molecular mechanism(s) by which phosphorylation of the alpha-subunit effects changes in channel activity are unknown. Inhibition of BK channels by PKA depends on phosphorylation of only a single alpha-subunit in the channel tetramer containing an alternatively spliced insert (STREX) suggesting that phosphorylation results in major conformational rearrangements of the C terminus. Here, we define the mechanism of PKA inhibition of BK channels and demonstrate that this regulation is conditional on the palmitoylation status of the channel. We show that the cytosolic C terminus of the STREX BK channel uniquely interacts with the plasma membrane via palmitoylation of evolutionarily conserved cysteine residues in the STREX insert. PKA phosphorylation of the serine residue immediately upstream of the conserved palmitoylated cysteine residues within STREX dissociates the C terminus from the plasma membrane, inhibiting STREX channel activity. Abolition of STREX palmitoylation by site-directed mutagenesis or pharmacological inhibition of palmitoyl transferases prevents PKA-mediated inhibition of BK channels. Thus, palmitoylation gates BK channel regulation by PKA phosphorylation. Palmitoylation and phosphorylation are both dynamically regulated; thus, cross-talk between these 2 major posttranslational signaling cascades provides a mechanism for conditional regulation of BK channels. Interplay of these distinct signaling cascades has important implications for the dynamic regulation of BK channels and physiological homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

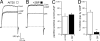
Similar articles
-
Palmitoylation of the S0-S1 linker regulates cell surface expression of voltage- and calcium-activated potassium (BK) channels.J Biol Chem. 2010 Oct 22;285(43):33307-33314. doi: 10.1074/jbc.M110.153940. Epub 2010 Aug 6. J Biol Chem. 2010. PMID: 20693285 Free PMC article.
-
Palmitoylation and membrane association of the stress axis regulated insert (STREX) controls BK channel regulation by protein kinase C.J Biol Chem. 2012 Sep 14;287(38):32161-71. doi: 10.1074/jbc.M112.386359. Epub 2012 Jul 29. J Biol Chem. 2012. PMID: 22843729 Free PMC article.
-
Multiple palmitoyltransferases are required for palmitoylation-dependent regulation of large conductance calcium- and voltage-activated potassium channels.J Biol Chem. 2010 Jul 30;285(31):23954-62. doi: 10.1074/jbc.M110.137802. Epub 2010 May 27. J Biol Chem. 2010. PMID: 20507996 Free PMC article.
-
S-acylation dependent post-translational cross-talk regulates large conductance calcium- and voltage- activated potassium (BK) channels.Front Physiol. 2014 Aug 5;5:281. doi: 10.3389/fphys.2014.00281. eCollection 2014. Front Physiol. 2014. PMID: 25140154 Free PMC article. Review.
-
Posttranscriptional and Posttranslational Regulation of BK Channels.Int Rev Neurobiol. 2016;128:91-126. doi: 10.1016/bs.irn.2016.02.012. Epub 2016 Mar 3. Int Rev Neurobiol. 2016. PMID: 27238262 Review.
Cited by
-
Large conductance Ca²⁺-activated K⁺ (BK) channels promote secretagogue-induced transition from spiking to bursting in murine anterior pituitary corticotrophs.J Physiol. 2015 Mar 1;593(5):1197-211. doi: 10.1113/jphysiol.2015.284471. Epub 2015 Jan 23. J Physiol. 2015. PMID: 25615909 Free PMC article.
-
Probing the lipid-protein interface using model transmembrane peptides with a covalently linked acyl chain.Biophys J. 2011 Oct 19;101(8):1959-67. doi: 10.1016/j.bpj.2011.09.020. Biophys J. 2011. PMID: 22004750 Free PMC article.
-
Palmitoylation of the β4-subunit regulates surface expression of large conductance calcium-activated potassium channel splice variants.J Biol Chem. 2013 May 3;288(18):13136-44. doi: 10.1074/jbc.M113.461830. Epub 2013 Mar 16. J Biol Chem. 2013. PMID: 23504458 Free PMC article.
-
The physiology of protein S-acylation.Physiol Rev. 2015 Apr;95(2):341-76. doi: 10.1152/physrev.00032.2014. Physiol Rev. 2015. PMID: 25834228 Free PMC article. Review.
-
Trafficking and function of the cystic fibrosis transmembrane conductance regulator: a complex network of posttranslational modifications.Am J Physiol Lung Cell Mol Physiol. 2016 Oct 1;311(4):L719-L733. doi: 10.1152/ajplung.00431.2015. Epub 2016 Jul 29. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27474090 Free PMC article. Review.
References
-
- Levitan IB. Adv Second Messenger Phosphoprotein Res. 1999;33:3–22. - PubMed
-
- Brenner R, et al. Vasoregulation by the beta 1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. - PubMed
-
- Sausbier M, et al. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005;112:60–68. - PubMed
-
- Brenner R, et al. BK channel beta 4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

