Nuclear export of the rate-limiting enzyme in phosphatidylcholine synthesis is mediated by its membrane binding domain
- PMID: 19098306
- PMCID: PMC2666183
- DOI: 10.1194/jlr.M800632-JLR200
Nuclear export of the rate-limiting enzyme in phosphatidylcholine synthesis is mediated by its membrane binding domain
Abstract
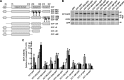
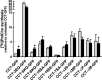
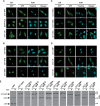
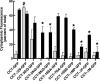
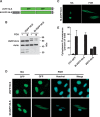
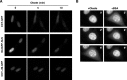
CTP:phosphocholine cytidylyltransferase alpha (CCTalpha), the rate-limiting enzyme in the CDP-choline pathway for phosphatidylcholine (PtdCho) synthesis, is activated by translocation to nuclear membranes. However, CCTalpha is cytoplasmic in cells with increased capacity for PtdCho synthesis and following acute activation, suggesting that nuclear export is linked to activation. The objective of this study was to identify which CCTalpha domains were involved in nuclear export in response to the lipid activators farnesol (FOH) and oleate. Imaging of CCT-green fluorescent protein (GFP) mutants expressed in CCTalpha-deficient CHO58 cells showed that FOH-mediated translocation to nuclear membranes and export to the cytoplasm required the membrane binding amphipathic helix (domain M). Nuclear export was reduced by a mutation that mimics constitutive phosphorylation of the CCT phosphorylation (P) domain. However, domain M alone was sufficient to promote translocation to the nuclear envelope and export of a nuclear-localized GFP construct in FOH- or oleate-treated CHO58 cells. In the context of acute activation with lipid mediators, nuclear export of CCT-GFP mutants correlated with in vitro activity but not PtdCho synthesis. This study describes a nuclear export pathway that is dependent on membrane interaction of an amphipathic helix, thus linking lipid-dependent activation to the nuclear/cytoplasmic distribution of CCTalpha.
Figures

References
-
- Vance J. E., and D. E. Vance. 2005. Metabolic insights into phospholipid function using gene-targeted mice. J. Biol. Chem. 280 10877–10880. - PubMed
-
- Cui Z., and M. Houweling. 2002. Phosphatidylcholine and cell death. Biochim. Biophys. Acta. 1585 87–96. - PubMed
-
- Vance D. E., Z. Li, and R. L. Jacobs. 2007. Hepatic phosphatidylethanolamine N-methyltransferase, unexpected roles in animal biochemistry and physiology. J. Biol. Chem. 282 33237–33241. - PubMed
-
- Lykidis A., and S. Jackowski. 2001. Regulation of mammalian cell membrane biosynthesis. Prog. Nucleic Acid Res. Mol. Biol. 65 361–393. - PubMed
-
- Karim M., P. Jackson, and S. Jackowski. 2003. Gene structure, expression and identification of a new CTP:phosphocholine cytidylyltransferase beta isoform. Biochim. Biophys. Acta. 1633 1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

