Localization and structure of the ankyrin-binding site on beta2-spectrin
- PMID: 19098307
- PMCID: PMC2652297
- DOI: 10.1074/jbc.M809245200
Localization and structure of the ankyrin-binding site on beta2-spectrin
Abstract
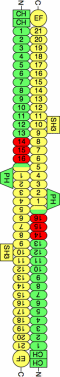
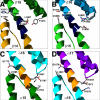
Spectrins are tetrameric actin-cross-linking proteins that form an elastic network, termed the membrane skeleton, on the cytoplasmic surface of cellular membranes. At the plasma membrane, the membrane skeleton provides essential support, preventing loss of membrane material to environmental shear stresses. The skeleton also controls the location, abundance, and activity of membrane proteins that are critical to cell and tissue function. The ability of the skeleton to modulate membrane stability and function requires adaptor proteins that bind the skeleton to membranes. The principal adaptors are the ankyrin proteins, which bind to the beta-subunit of spectrin and to the cytoplasmic domains of numerous integral membrane proteins. Here, we present the crystal structure of the ankyrin-binding domain of human beta2-spectrin at 1.95 A resolution together with mutagenesis data identifying the binding surface for ankyrins on beta2-spectrin.
Figures




References
-
- Mohandas, N., Chasis, J. A., and Shohet, S. B. (1983) Semin. Hematol. 20 225-242 - PubMed
-
- Bennett, V., and Baines, A. J. (2001) Physiol. Rev. 81 1353-1392 - PubMed
-
- Mohler, P. J., and Bennett, V. (2005) Front. Biosci. 10 2832-2840 - PubMed
-
- Bennett, V., and Healy, J. (2008) Trends Mol. Med. 14 28-36 - PubMed
-
- Rief, M., Pascual, J., Saraste, M., and Gaub, H. E. (1999) J. Mol. Biol. 286 553-561 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

