Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice
- PMID: 19098310
- PMCID: PMC2652279
- DOI: 10.1074/jbc.M805890200
Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice
Abstract
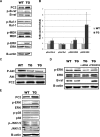
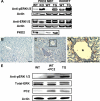
The pathogenic mechanisms of human autosomal dominant polycystic kidney disease (ADPKD) have been well known to include the mutational inactivation of PKD2. Although haploinsufficiency and loss of heterozygosity at the Pkd2 locus can cause cyst formation in mice, polycystin-2 is frequently expressed in the renal cyst of human ADPKD, raising the possibility that deregulated activation of PKD2 may be associated with the cystogenesis of human ADPKD. To determine whether increased PKD2 expression is physiologically pathogenic, we generated PKD2-overexpressing transgenic mice. These mice developed typical renal cysts and an increase of proliferation and apoptosis, which are reflective of the human ADPKD phenotype. These manifestations were first observed at six months, and progressed with age. In addition, we found that ERK activation was induced by PKD2 overexpression via B-Raf signaling, providing a possible molecular mechanism of cystogenesis. In PKD2 transgenic mice, B-Raf/MEK/ERK sequential signaling was up-regulated. Additionally, the transgenic human polycystin-2 partially rescues the lethality of Pkd2 knock-out mice and therefore demonstrates that the transgene generated a functional product. Functional strengthening or deregulated activation of PKD2 may be a direct cause of ADPKD. The present study provides evidence for an in vivo role of overexpressed PKD2 in cyst formation. This transgenic mouse model should provide new insights into the pathogenic mechanism of human ADPKD.
Figures






References
-
- Gabow, P. A. (1993) N. Engl. J. Med. 329 332-342 - PubMed
-
- Grantham, J. J. (1996) Am. J. Kidney Dis. 28 788-803 - PubMed
-
- Carone, F. A., Bacallao, R., and Kanwar, Y. S. (1996) The etiology, pathogenesis, and treatment of autosomal dominant polycystic kidney disease: recent advances, pp. 111-124, Oxford University Press, Oxford, UK
-
- Peters, D. J., and Sandkuijl, L. A. (1992) Contrib. Nephrol. 97 128-139 - PubMed
-
- Roscoe, J. M., Brissenden, J. E., Williams, E. A., Chery, A. L., and Silverman, M. (1993) Kidney Int. 44 1101-1108 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

