HMG-CoA reductase inhibitors (statin) prevents retinal neovascularization in a model of oxygen-induced retinopathy
- PMID: 19098312
- PMCID: PMC2819291
- DOI: 10.1167/iovs.08-2158
HMG-CoA reductase inhibitors (statin) prevents retinal neovascularization in a model of oxygen-induced retinopathy
Abstract
Purpose: Retinal neovascularization (RNV) is a primary cause of blindness and involves the dysfunction of retinal capillaries. Recent studies have emphasized the beneficial effects of inhibitors of HMG-CoA reductase (statins) in preventing vascular dysfunction. In the present study, the authors characterized the therapeutic effects of statins on RNV.
Methods: Statin treatment (10 mg/kg/d fluvastatin) was tested in a mouse model of oxygen-induced retinopathy. Morphometric analysis was conducted to determine the extent of capillary growth. Pimonidazole hydrochloride was used to assess retinal ischemia. Western blot and immunohistochemical analyses were used to assess protein expression levels and immunolocalization. Lipid peroxidation and superoxide radical formation were determined to assess oxidative changes.
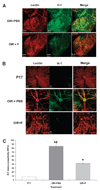
Results: Fluvastatin treatment significantly reduced the area of the capillary-free zone (P < 0.01), decreased the formation of neovascular tufts (P < 0.01), and ameliorated retinal ischemia. These morphologic and functional changes were associated with statin effects in preventing the upregulation of VEGF, HIF-1 alpha, phosphorylated STAT3, and vascular expression of the inflammatory mediator ICAM-1 (P < 0.01). Superoxide production and lipid peroxidation in the ischemic retina were also reduced by statin treatment (P < 0.01).
Conclusions: These data suggest the beneficial effects of statin treatment in preventing retinal neovascularization. These beneficial effects appear to result from the anti-oxidant and anti-inflammatory properties of statins.
Figures




Similar articles
-
Trans-Chalcone prevents VEGF expression and retinal neovascularization in the ischemic retina.Exp Eye Res. 2011 Oct;93(4):350-4. doi: 10.1016/j.exer.2011.02.007. Epub 2011 Feb 24. Exp Eye Res. 2011. PMID: 21354136
-
Aldose reductase deficiency reduced vascular changes in neonatal mouse retina in oxygen-induced retinopathy.Invest Ophthalmol Vis Sci. 2012 Aug 20;53(9):5698-712. doi: 10.1167/iovs.12-10122. Invest Ophthalmol Vis Sci. 2012. PMID: 22836764
-
Silencing of S100A4, a metastasis-associated protein, inhibits retinal neovascularization via the downregulation of BDNF in oxygen-induced ischaemic retinopathy.Eye (Lond). 2016 Jun;30(6):877-87. doi: 10.1038/eye.2016.43. Epub 2016 Mar 18. Eye (Lond). 2016. PMID: 26987588 Free PMC article.
-
Hyaloid Vasculature as a Major Source of STAT3+ (Signal Transducer and Activator of Transcription 3) Myeloid Cells for Pathogenic Retinal Neovascularization in Oxygen-Induced Retinopathy.Arterioscler Thromb Vasc Biol. 2020 Dec;40(12):e367-e379. doi: 10.1161/ATVBAHA.120.314567. Epub 2020 Oct 29. Arterioscler Thromb Vasc Biol. 2020. PMID: 33115265
-
Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice.J Pineal Res. 2018 May;64(4):e12473. doi: 10.1111/jpi.12473. Epub 2018 Mar 8. J Pineal Res. 2018. PMID: 29411894
Cited by
-
Angiotensin II type 1 receptor antagonist prevents hepatic carcinoma in rats with nonalcoholic steatohepatitis.J Gastroenterol. 2013 Apr;48(4):491-503. doi: 10.1007/s00535-012-0651-7. Epub 2012 Aug 14. J Gastroenterol. 2013. PMID: 22886508
-
NADPH oxidase, NOX1, mediates vascular injury in ischemic retinopathy.Antioxid Redox Signal. 2014 Jun 10;20(17):2726-40. doi: 10.1089/ars.2013.5357. Epub 2013 Oct 30. Antioxid Redox Signal. 2014. PMID: 24053718 Free PMC article.
-
Erythropoietin receptor antibody inhibits oxidative stress induced retinal neovascularization in mice.Int J Ophthalmol. 2011;4(3):243-6. doi: 10.3980/j.issn.2222-3959.2011.03.05. Epub 2011 Jun 18. Int J Ophthalmol. 2011. PMID: 22553653 Free PMC article.
-
Vascular and Neuronal Protection in the Developing Retina: Potential Therapeutic Targets for Retinopathy of Prematurity.Int J Mol Sci. 2019 Sep 3;20(17):4321. doi: 10.3390/ijms20174321. Int J Mol Sci. 2019. PMID: 31484463 Free PMC article. Review.
-
Multi-modal proteomic analysis of retinal protein expression alterations in a rat model of diabetic retinopathy.PLoS One. 2011 Jan 13;6(1):e16271. doi: 10.1371/journal.pone.0016271. PLoS One. 2011. PMID: 21249158 Free PMC article.
References
-
- Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19(6):442–455. - PubMed
-
- Shama N, Inachulev T. Role of vascular endothelial growth factor in ocular angiogenesis. Ophthalmol Clin North Am. 2006;19(3):335–344. - PubMed
-
- Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6(4):511–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

