Sarcoplasmic reticulum-mitochondrial symbiosis: bidirectional signaling in skeletal muscle
- PMID: 19098522
- PMCID: PMC2740713
- DOI: 10.1097/JES.0b013e3181911fa4
Sarcoplasmic reticulum-mitochondrial symbiosis: bidirectional signaling in skeletal muscle
Abstract
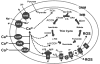
In mammalian skeletal muscle, an intimate association between the sarcoplasmic reticulum (SR) and mitochondria results in a symbiotic and privileged bidirectional communication between these organelles. Orthograde signaling reflects SR calcium (Ca) release stimulating mitochondrial adenosine triphosphate production via excitation-metabolism coupling. Retrograde signaling involves mitochondrial inhibition of local SR Ca release by controlling the redox environment of the Ca release unit.
Figures



Similar articles
-
Sarcoplasmic reticulum-mitochondrial through-space coupling in skeletal muscle.Appl Physiol Nutr Metab. 2009 Jun;34(3):389-95. doi: 10.1139/H09-044. Appl Physiol Nutr Metab. 2009. PMID: 19448704 Free PMC article.
-
Activation and propagation of Ca2+ release from inside the sarcoplasmic reticulum network of mammalian skeletal muscle.J Physiol. 2014 Sep 1;592(17):3727-46. doi: 10.1113/jphysiol.2014.274274. Epub 2014 Jun 27. J Physiol. 2014. PMID: 24973406 Free PMC article.
-
Physical and Functional Cross Talk Between Endo-Sarcoplasmic Reticulum and Mitochondria in Skeletal Muscle.Antioxid Redox Signal. 2020 Apr 20;32(12):873-883. doi: 10.1089/ars.2019.7934. Epub 2019 Dec 11. Antioxid Redox Signal. 2020. PMID: 31825235 Review.
-
Preserved Ca2+ handling and excitation-contraction coupling in muscle fibres from diet-induced obese mice.Diabetologia. 2020 Nov;63(11):2471-2481. doi: 10.1007/s00125-020-05256-8. Epub 2020 Aug 25. Diabetologia. 2020. PMID: 32840676
-
Impaired calcium release during fatigue.J Appl Physiol (1985). 2008 Jan;104(1):296-305. doi: 10.1152/japplphysiol.00908.2007. Epub 2007 Oct 25. J Appl Physiol (1985). 2008. PMID: 17962573 Review.
Cited by
-
Growth Hormone Secretagogues and the Regulation of Calcium Signaling in Muscle.Int J Mol Sci. 2019 Sep 5;20(18):4361. doi: 10.3390/ijms20184361. Int J Mol Sci. 2019. PMID: 31491959 Free PMC article. Review.
-
Endurance exercise attenuates juvenile irradiation-induced skeletal muscle functional decline and mitochondrial stress.Skelet Muscle. 2022 Apr 12;12(1):8. doi: 10.1186/s13395-022-00291-y. Skelet Muscle. 2022. PMID: 35414122 Free PMC article.
-
Doxorubicin causes lesions in the electron transport system of skeletal muscle mitochondria that are associated with a loss of contractile function.J Biol Chem. 2019 Dec 20;294(51):19709-19722. doi: 10.1074/jbc.RA119.008426. Epub 2019 Nov 5. J Biol Chem. 2019. PMID: 31690631 Free PMC article.
-
Antioxidant Treatment Reduces Formation of Structural Cores and Improves Muscle Function in RYR1Y522S/WT Mice.Oxid Med Cell Longev. 2017;2017:6792694. doi: 10.1155/2017/6792694. Epub 2017 Sep 10. Oxid Med Cell Longev. 2017. PMID: 29062463 Free PMC article.
-
Excitation-contraction coupling in mammalian skeletal muscle: Blending old and last-decade research.Front Physiol. 2022 Sep 2;13:989796. doi: 10.3389/fphys.2022.989796. eCollection 2022. Front Physiol. 2022. PMID: 36117698 Free PMC article. Review.
References
-
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: Cellular mechanisms. Physiol Rev. 2008;88(1):287–332. - PubMed
-
- Balaban RS. Cardiac energy metabolism homeostasis: Role of cytosolic calcium. J Mol Cell Cardiol. 2002;34(10):1259–71. - PubMed
-
- Boncompagni S, Protasi F. Tethers: Structural connections between SR and the outer mitochondrial membrane. Biophys J. 2007;92:313a. - PubMed
-
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–C33. - PubMed
-
- Byrd SK. Alterations in the sarcoplasmic reticulum: A possible link to exercise-induced muscle damage. Med Sci Sports Exerc. 1992;24(5):531–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

