Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes
- PMID: 19098898
- PMCID: PMC2895694
- DOI: 10.1038/nbt.1515
Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes
Abstract
Delivery of genes to the brain and spinal cord across the blood-brain barrier (BBB) has not yet been achieved. Here we show that adeno-associated virus (AAV) 9 injected intravenously bypasses the BBB and efficiently targets cells of the central nervous system (CNS). Injection of AAV9-GFP into neonatal mice through the facial vein results in extensive transduction of dorsal root ganglia and motor neurons throughout the spinal cord and widespread transduction of neurons throughout the brain, including the neocortex, hippocampus and cerebellum. In adult mice, tail vein injection of AAV9-GFP leads to robust transduction of astrocytes throughout the entire CNS, with limited neuronal transduction. This approach may enable the development of gene therapies for a range of neurodegenerative diseases, such as spinal muscular atrophy, through targeting of motor neurons, and amyotrophic lateral sclerosis, through targeting of astrocytes. It may also be useful for rapid postnatal genetic manipulations in basic neuroscience studies.
Figures
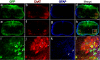
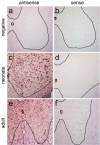


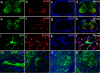
Comment in
-
Crossing the rubicon.Nat Biotechnol. 2009 Jan;27(1):42-4. doi: 10.1038/nbt0109-42. Nat Biotechnol. 2009. PMID: 19131996 Free PMC article.
-
The neonatal blood-brain barrier is functionally effective, and immaturity does not explain differential targeting of AAV9.Nat Biotechnol. 2009 Sep;27(9):804-5; author reply 805. doi: 10.1038/nbt0909-804. Nat Biotechnol. 2009. PMID: 19741632 No abstract available.
References
-
- Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002;1:131–139. - PubMed
-
- Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. - PubMed
-
- Azzouz M, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. - PubMed
-
- Ralph GS, et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

