The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells
- PMID: 19098919
- PMCID: PMC2742982
- DOI: 10.1038/ni.1690
The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells
Abstract
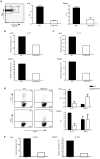
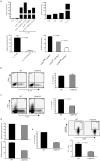
The inducible costimulatory molecule ICOS has been suggested to be important in the development of interleukin 17 (IL-17)-producing helper T cells (T(H)-17 cells) and of follicular helper T cells (T(FH) cells). Here we show that ICOS-deficient mice had no defect in T(H)-17 differentiation but had fewer T(H)-17 cells after IL-23 stimulation and fewer T(FH) cells. We also show that T(FH) cells produced IL-17 and that T(FH) cells in ICOS-deficient mice were defective in IL-17 production. Both T(H)-17 and T(FH) cells had higher expression of the transcription factor c-Maf. Genetic loss of c-Maf resulted in a defect in IL-21 production and fewer T(H)-17 and T(FH) cells. Thus our data suggest that ICOS-induced c-Maf regulates IL-21 production that in turn regulates the expansion of T(H)-17 and T(FH) cells.
Figures




Comment in
-
Helping and harming have something in common.Nat Immunol. 2009 Feb;10(2):138-40. doi: 10.1038/ni0209-138. Nat Immunol. 2009. PMID: 19148197 No abstract available.
References
-
- Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. - PubMed
-
- Matusevicius D, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. - PubMed
-
- Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246–1251. - PubMed
-
- Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01NS38037-04/NS/NINDS NIH HHS/United States
- 2R01NS35685-06/NS/NINDS NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- 1P01AI56299/AI/NIAID NIH HHS/United States
- R01 NS045937/NS/NINDS NIH HHS/United States
- R37 AI38310/AI/NIAID NIH HHS/United States
- R01 NS035685/NS/NINDS NIH HHS/United States
- 2R37NS30843-11/NS/NINDS NIH HHS/United States
- R37 AI038310/AI/NIAID NIH HHS/United States
- 1R01AI44880-03/AI/NIAID NIH HHS/United States
- R01 AI044880/AI/NIAID NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- R37 NS030843/NS/NINDS NIH HHS/United States
- 2P01A139671-07/PHS HHS/United States
- 1R01NS045937-01/NS/NINDS NIH HHS/United States
- R01 NS046414/NS/NINDS NIH HHS/United States
- 1R01NS046414/NS/NINDS NIH HHS/United States
- P01 NS038037/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

