The role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritis
- PMID: 19098927
- PMCID: PMC4433145
- DOI: 10.1038/ncprheum0961
The role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritis
Abstract
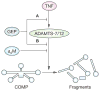
Loss of articular cartilage caused by extracellular matrix breakdown is the hallmark of arthritis. Degradative fragments of cartilage oligomeric matrix protein (COMP) have been observed in arthritic patients. ADAMTS-7 and ADAMTS-12, two members of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family, have been associated with COMP degradation in vitro, and are significantly overexpressed in the cartilage and synovium of patients with rheumatoid arthritis. Recent studies have demonstrated the importance of COMP degradation by ADAMTS-7 and ADAMTS-12. Specifically, the size of COMP fragments generated by ADAMTS-7 or ADAMTS-12 is similar to that of COMP-degradative fragments seen in arthritic patients. In addition, antibodies against ADAMTS-7 or ADAMTS-12 dramatically inhibit tumor necrosis factor-induced and interleukin-1beta-induced COMP degradation in cartilage explants. Furthermore, suppression of ADAMTS-7 or ADAMTS-12 expression using the small interfering RNA silencing approach in human chondrocytes markedly prevents COMP degradation. COMP degradation mediated by ADAMTS-7 and ADAMTS-12 is inhibited by alpha(2)-macroglobulin. More significantly, granulin-epithelin precursor, a newly characterized chondrogenic growth factor, disturbs the interaction between COMP and ADAMTS-7 and ADAMTS-12, preventing COMP degradation by these enzymes. This Review summarizes the evidence demonstrating that ADAMTS-7 and ADAMTS-12 are newly identified enzymes responsible for COMP degradation in arthritis, and that alpha(2)-macroglobulin and granulin-epithelin precursor represent their endogenous inhibitors.
Conflict of interest statement
The author declared no competing interests.
Figures
References
-
- Salzet M. Leech thrombin inhibitors. Curr Pharm Des. 2002;8:493–503. - PubMed
-
- Hedbom E, et al. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267:6132–6136. - PubMed
-
- Briggs MD, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10:330–336. - PubMed
-
- Cohn DH, et al. Mutations in the cartilage oligomeric matrix protein (COMP) gene in pseudoachondroplasia and multiple epiphyseal dysplasia. Ann NY Acad Sci. 1996;785:188–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
