Role of Cajal bodies and nucleolus in the maturation of the U1 snRNP in Arabidopsis
- PMID: 19098980
- PMCID: PMC2600615
- DOI: 10.1371/journal.pone.0003989
Role of Cajal bodies and nucleolus in the maturation of the U1 snRNP in Arabidopsis
Abstract
Background: The biogenesis of spliceosomal snRNPs takes place in both the cytoplasm where Sm core proteins are added and snRNAs are modified at the 5' and 3' termini and in the nucleus where snRNP-specific proteins associate. U1 snRNP consists of U1 snRNA, seven Sm proteins and three snRNP-specific proteins, U1-70K, U1A, and U1C. It has been shown previously that after import to the nucleus U2 and U4/U6 snRNP-specific proteins first appear in Cajal bodies (CB) and then in splicing speckles. In addition, in cells grown under normal conditions U2, U4, U5, and U6 snRNAs/snRNPs are abundant in CBs. Therefore, it has been proposed that the final assembly of these spliceosomal snRNPs takes place in this nuclear compartment. In contrast, U1 snRNA in both animal and plant cells has rarely been found in this nuclear compartment.
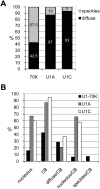
Methodology/principal findings: Here, we analysed the subnuclear distribution of Arabidopsis U1 snRNP-specific proteins fused to GFP or mRFP in transiently transformed Arabidopsis protoplasts. Irrespective of the tag used, U1-70K was exclusively found in the nucleus, whereas U1A and U1C were equally distributed between the nucleus and the cytoplasm. In the nucleus all three proteins localised to CBs and nucleoli although to different extent. Interestingly, we also found that the appearance of the three proteins in nuclear speckles differ significantly. U1-70K was mostly found in speckles whereas U1A and U1C in approximately 90% of cells showed diffuse nucleoplasmic in combination with CBs and nucleolar localisation.
Conclusions/significance: Our data indicate that CBs and nucleolus are involved in the maturation of U1 snRNP. Differences in nuclear accumulation and distribution between U1-70K and U1A and U1C proteins may indicate that either U1-70K or U1A and U1C associate with, or is/are involved, in other nuclear processes apart from pre-mRNA splicing.
Conflict of interest statement
Figures




References
-
- Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nature Rev Mol Cell Biol. 2003;4:605–612. - PubMed
-
- Stanĕk D, Neugebauer KM. The Cajal body: a meeting place for spliceosomal snRNPs in the nuclear maze. Chromosoma. 2006;115:343–354. - PubMed
-
- Gall JG. Cajal bodies: The first 100 years. Annu Rev Cell Dev Biol. 2000;16:273–300. - PubMed
-
- Cioce M, Lamond AI. Cajal bodies: a long history of discovery. Annu Rev Cell Dev Biol. 2005;21:105–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

