Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect
- PMID: 19099374
- PMCID: PMC3190292
- DOI: 10.1089/scd.2008.0253
Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect
Abstract
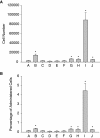
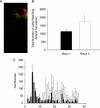
Intravenous (IV) stem cell delivery for regenerative tissue therapy has been increasingly used in both experimental and clinical trials. However, recent data suggest that the majority of administered stem cells are initially trapped in the lungs. We sought to investigate variables that may affect this pulmonary first-pass effect. In anesthetized Sprague-Dawley rats, silicone tubing catheters were placed in the left internal jugular vein and common carotid artery. We investigated four different cell types: mesenchymal stromal cells (MSC), multipotent adult progenitor cells (MAPCs), bone marrow-derived mononuclear cells (BMMC), and neural stem cells (NSC). Cells were co-labeled with Qtracker 655 (for flow cytometry) and Qtracker 800 (for infrared imaging) and infused intravenously with continual arterial sample collection. Samples were analyzed via flow cytometry to detect labeled cells reaching the arterial circulation. Following sampling and exsanguination, heart, lungs, spleen, kidney, and liver were harvested and placed on an infrared imaging system to identify the presence of labeled cells. The majority of MSCs were trapped inside the lungs following intravenous infusion. NSC and MAPC pulmonary passage was 2-fold and BMMC passage was 30-fold increased as compared to MSCs. Inhibition of MSC CD49d significantly increased MSC pulmonary passage. Infusion via two boluses increased pulmonary MSC passage as compared to single bolus administration. Infrared imaging revealed stem cells evenly distributed over all lung fields. Larger stem and progenitor cells are initially trapped inside the lungs following intravenous administration with a therapeutically questionable number of cells reaching the arterial system acutely.
Figures




References
-
- Dimmeler S. Burchfield J. Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008 Feb;28:208–216. - PubMed
-
- Ott HC. McCue J. Taylor DA. Cell-based cardiovascular repair—the hurdles and the opportunities. Basic Res Cardiol. 2005 Nov;100:504–517. - PubMed
-
- Sherman W. Martens TP. Viles-Gonzalez JF. Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Pract. 2006 Mar;3:S57–S64. - PubMed
-
- Mahmood A. Lu D. Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004 Nov;55:1185–1193. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

