Urine metabolites reflect time-dependent effects of cyclosporine and sirolimus on rat kidney function
- PMID: 19099400
- PMCID: PMC2646011
- DOI: 10.1021/tx800253x
Urine metabolites reflect time-dependent effects of cyclosporine and sirolimus on rat kidney function
Abstract
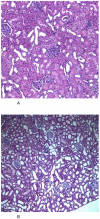
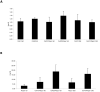
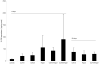
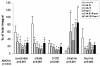
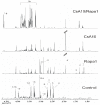
The clinical use of the immunosuppressant calcineurin inhibitor cyclosporine is limited by its nephrotoxicity. This is enhanced when combined with the immunosuppressive mTOR inhibitor sirolimus. Nephrotoxicity of both drugs is not yet fully understood. The goal was to gain more detailed mechanistic insights into the time-dependent effects of cyclosporine and sirolimus on the rat kidney by using a comprehensive approach including metabolic profiling in urine ((1)H NMR spectroscopy), kidney histology, kidney function parameters in plasma, measurement of glomerular filtration rates, the oxidative stress marker 15-F(2t)-isoprostane in urine, and immunosuppressant concentrations in blood and kidney. Male Wistar rats were treated with vehicle (controls), cyclosporine (10/25 mg/kg/day), and/or sirolimus (1 mg/kg/day) by oral gavage once daily for 6 and 28 days. Twenty-eight day treatment led to a decrease of glomerular filtration rates (cyclosporine, -59%; sirolimus, -25%). These were further decreased when both drugs were combined (-86%). Histology revealed tubular damage after treatment with cyclosporine, which was enhanced when sirolimus was added. No other part of the kidney was affected. (1)H NMR spectroscopy analysis of urine (day 6) revealed time-dependent changes of 2-oxoglutarate, citrate, and succinate concentrations. In combination with increased urine isoprostane concentrations, these changes indicated oxidative stress. After 28 days of cyclosporine treatment, urine metabonomics shifted to patterns typical for proximal tubular damage with reduction of Krebs cycle intermediates and trimethylamine-N-oxide concentrations, whereas acetate, lactate, trimethylamine, and glucose concentrations increased. Again, sirolimus enhanced these negative effects. Our results indicate that cyclosporine and/or sirolimus induce damage of the renal tubular system. This is reflected by urine metabolite patterns, which seem to be more sensitive than currently used clinical kidney function markers such as creatinine concentrations in serum. Metabolic profiling in urine may provide the basis for the development of toxicodynamic monitoring strategies for immunosuppressant nephrotoxicity.
Figures
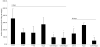


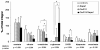
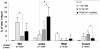

References
-
- Meier-Kriesche HU, Hricik DE. Are we ready to give up on calcineurin inhibitors? Am J Transplant. 2006;6:445–446. - PubMed
-
- Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. - PubMed
-
- Shihab FS. Cyclosporine nephropathy: pathophysiology and clinical impact. Semin Nephrol. 1996;16:536–547. - PubMed
-
- Guerra G, Srinivas TR, Meier-Kriesche HU. Calcineurin inhibitor-free immunosuppression in kidney transplantation. Transpl Int. 2007;20:813–827. - PubMed
-
- Campistol JM, Grinyo JM. Exploring treatment options in renal transplantation: the problems of chronic allograft dysfunction and drug-related nephrotoxicity. Transplantation. 2001;71:SS42–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

