C-H bond functionalization via hydride transfer: Lewis acid catalyzed alkylation reactions by direct intramolecular coupling of sp3 C-H bonds and reactive alkenyl oxocarbenium intermediates
- PMID: 19099474
- PMCID: PMC2954885
- DOI: 10.1021/ja806068h
C-H bond functionalization via hydride transfer: Lewis acid catalyzed alkylation reactions by direct intramolecular coupling of sp3 C-H bonds and reactive alkenyl oxocarbenium intermediates
Abstract
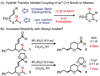
C-H bond functionalization enables strategically new approaches to the synthesis of complex organic molecules including biologically active compounds, research probes and functional organic materials. To address the shortcomings of transition metal catalyzed processes, we have developed a new approach to direct coupling of sp(3) C-H bonds and alkenes based on Lewis acid-promoted hydride transfer. Activation of alpha,beta-unsaturated aldehydes and ketones with Lewis acid triggers intramolecular hydride transfer, leading to a zwitterionic intermediate, which in turn undergoes ionic cyclization to afford the cyclic alkylation product. The scope of this method is expanded by the generation of alkenyl-oxocarbenium species as highly activated alkene intermediates capable of abstracting a hydride from unreactive carbon centers, including benzyl-, allyl-, and crotyl-ethers, as well as primary alkyl ethers, at room temperature. The alkenyl acetal and ketal substrates show dramatically faster rates of cyclization, as well as improved chemical yield and diastereoselectivity, compared to the corresponding carbonyl compounds. Furthermore, the use of boron trifluoride etherate as the Lewis acid and ethylene glycol as the organocatalyst provides a highly active catalytic system, presumably via the in situ formation of alkenyl-oxocarbenium intermediates, which eliminates the need for expensive transition metal Lewis acids or the preparation of ketal substrates. This binary catalytic system greatly improves the efficiency of the hydride transfer-initiated alkylation reactions.
Figures


References
-
- Pastine SJ, McQuaid KM, Sames D. J. Am. Chem. Soc. 2005;127:12180–12181. - PubMed
-
-
The tert-amino effect cyclizations: Verboom W, Reinhoudt DN, Visser R, Harkema S. J. Org. Chem. 1984;49:269–276.. Recent review: Mátyus P, Éliás O, Tapolcsányi P, Polonka-Bálint A, Halász-Dajka B. Synthesis. 2006;16:2625–2639.. Formation of carbocycles: Atkinson RS, Green RH. J. Chem. Soc, Perkin Tans. 1974;1:401..
-
-
-
Gassman PG, Singleton DA, Wilwerding JJ, Chavan SP. J. Am. Chem. Soc. 1987;109:2182–2184. Roush WR, Gillis HR, Essenfeld AP. J. Org. Chem. 1984;49:4674–4682.. Review: Harmata M, Rashatasakhon P. Tetrahedron. 2003;59:2371–2395..
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

