A randomized trial on effectiveness of artemether-lumefantrine versus artesunate plus amodiaquine for unsupervised treatment of uncomplicated Plasmodium falciparum malaria in Ghanaian children
- PMID: 19099594
- PMCID: PMC2625364
- DOI: 10.1186/1475-2875-7-261
A randomized trial on effectiveness of artemether-lumefantrine versus artesunate plus amodiaquine for unsupervised treatment of uncomplicated Plasmodium falciparum malaria in Ghanaian children
Abstract
Background: Numerous trials have demonstrated high efficacy and safety of artemisinin-based combination therapy (ACT) under supervised treatment. In contrast, effectiveness studies comparing different types of ACT applied unsupervised are scarce. The aim of this study was to compare effectiveness, tolerability and acceptance of artesunate plus amodiaquine (ASAQ) against that of artemether-lumefantrine (AL) in Ghanaian children with uncomplicated Plasmodium falciparum malaria.
Methods: A randomized open-label trial was conducted at two district hospitals in the Ashanti region, Ghana, an area of intense malaria transmission. A total of 246 children under five years of age were randomly assigned to either ASAQ (Arsucam) or AL (Coartem). Study participants received their first weight-adjusted dose under supervision. After the parent/guardian was advised of times and mode of administration the respective three-day treatment course was completed unobserved at home. Follow-up visits were performed on days 3, 7, 14 and 28 to evaluate clinical and parasitological outcomes, adverse events, and haematological recovery. Length polymorphisms of variable regions of msp1 and msp2 were determined to differentiate recrudescences from reinfections. Acceptance levels of both treatment regimens were assessed by means of standardized interviews.
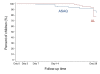
Results: Adequate clinical and parasitological responses after AL and ASAQ treatment were similar (88.3% and 91.7%, respectively). Interestingly, more late clinical failures until day 28 occurred in AL-treated children than in those who received ASAQ (17.5% and 7.3%, respectively; Hazard Ratio 2.41, 95% CI 1.00-5.79, p < 0.05).Haematological recovery and drug tolerability were not found to be significantly different in both study arms. The acceptance of treatment with ASAQ was higher than that with AL (rank-scores 10.6 and 10.3, respectively; p < 0.05).
Conclusion: Unobserved AL and ASAQ treatment showed high adequate clinical and parasitological responses, though AL was inferior in preventing late clinical failures.
Figures

References
-
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, Kokwaro G, Ouma J, Hien TT, Molyneux ME, Taylor TE, Newbold CI, Ruebush TK, 2nd, Danis M, Greenwood BM, Anderson RM, Olliaro P. Averting a malaria disaster. Lancet. 1999;353:1965–1967. doi: 10.1016/S0140-6736(98)07367-X. - DOI - PubMed
-
- Nosten F, van Vugt M, Price R, Luxemburger C, Thway KL, Brockman A, McGready R, ter Kuile F, Looareesuwan S, White NJ. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. doi: 10.1016/S0140-6736(00)02505-8. - DOI - PubMed
-
- Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64:1–11. - PubMed
-
- World Health Organization Antimalarial drug combination therapy: report of a WHO Technical Consultation Geneva. 2001. Document no. WHO/CDS/RBM/2001.35.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

