The urge-to-cough and cough motor response modulation by the central effects of nicotine
- PMID: 19100331
- PMCID: PMC3121142
- DOI: 10.1016/j.pupt.2008.11.013
The urge-to-cough and cough motor response modulation by the central effects of nicotine
Abstract
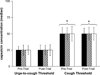
The urge-to-cough is a respiratory sensation that precedes the cough motor response. Since affective state modulates the perception of respiratory sensations such as dyspnoea, we wanted to test whether nicotine, an anxiolytic, would modulate the urge-to-cough and hence, the cough motor response. We hypothesized that withdrawal from and administration of nicotine in smoking subjects would modulate their anxiety levels, urge-to-cough and cough motor response to capsaicin stimulation. Twenty smoking (SM) adults (8F, 12M; 22+/-3 years; 2.9+/-2.0 pack years) and matched non-smoking (NS) controls (22+/-2 years) were presented with randomized concentrations of capsaicin (0-200 microM) before and after nicotine (SM only) gum and/or placebo (SM and NS) gum. Subjects rated their urge-to-cough using a Borg scale at the end of each capsaicin presentation. Cough number and cough motor pattern were determined from airflow tracings. Subjects completed State-Trait Anxiety Inventory (STAI) questionnaires before and after gum administration. SM subjects that withdrew from cigarette smoking for 12 h exhibited an increase in anxiety scores, a greater number of coughs and higher urge-to-cough ratings compared to NS subjects. Administration of nicotine gum reduced anxiety scores, cough number and urge-to-cough ratings to match the NS subjects. There was no effect of placebo gum on any of the measured parameters in the SM and NS groups. The results from this study suggest that modulation of the central neural state with nicotine withdrawal and administration in young smoking adults is associated with a change in anxiety levels which in turn modulates the perceptual and motor response to irritant cough stimulants.
Figures







References
-
- Davenport PW. Urge-to-cough: what can it teach us about cough? Lung. 2008;186(Suppl. 1):S107–S111. - PubMed
-
- Davenport PW. Clinical cough I: the urge-to-cough: a respiratory sensation. In: Chung KF, Widdicombe JG, editors. Handbook of experimental pharmacology: pharmacology and therapeutics of cough. Heidelberg: Springer; 2009. pp. 263–276. - PubMed
-
- Davenport PW, Sapeinza CM, Bolser DC. Psychophysical assessment of the urge-to-cough. Eur Respir J. 2002;12:249–253.
-
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in dyspnea. Respir Physiol Neurobiol. in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

