IL-12 p40 homodimer, but not IL-12 p70, induces the expression of IL-16 in microglia and macrophages
- PMID: 19100623
- PMCID: PMC2660888
- DOI: 10.1016/j.molimm.2008.10.033
IL-12 p40 homodimer, but not IL-12 p70, induces the expression of IL-16 in microglia and macrophages
Abstract
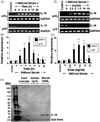
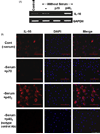
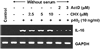
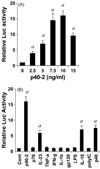
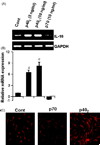
IL-16, a leukocyte chemoattractant factor (LCF), is involved in the disease process of multiple sclerosis and other autoimmune disorders. However, mechanisms by which this LCF is expressed are poorly understood. The present study underlines the importance of IL-12 p40 homodimer (p40(2)), the so-called biologically inactive molecule, in inducing the expression of IL-16 in primary mouse and human microglia, mouse BV-2 microglial cells, mouse peritoneal macrophages, and RAW264.7 cells. In contrast, IL-12 p70, the bioactive heterodimeric cytokine, was unable to induce the expression of IL-16 in any of these cell types. Similarly IL-12 p40(2) also induced the activation of IL-16 promoter in microglia. Among various stimuli tested, p40(2) was the most potent one followed by p40 monomer, IL-16 and IL-23 in inducing the activation of IL-16 promoter in microglial cells. Furthermore, induction of IL-16 mRNA expression by over-expression of p40, but not p35, cDNA and induction of IL-16 expression by p40(2) in microglia isolated from IL-12p35 (-/-) mice confirm that p40, but not p35, is responsible for the induction of IL-16. Finally, by using primary microglia isolated from IL-12Rbeta1 (-/-) and IL-12Rbeta2 (-/-) mice, we demonstrate that p40(2) induces the expression of this LCF via IL-12Rbeta1 but not IL-12Rbeta2. These results delineate a novel biological function of p40(2) and raise the possibility that biological function of IL-12 p40(2) may be different from IL-12 p70.
Figures





Similar articles
-
Interleukin-12 (IL-12), but not IL-23, induces the expression of IL-7 in microglia and macrophages: implications for multiple sclerosis.Immunology. 2014 Apr;141(4):549-63. doi: 10.1111/imm.12214. Immunology. 2014. PMID: 24224652 Free PMC article.
-
Induction of lymphotoxin-alpha by interleukin-12 p40 homodimer, the so-called biologically inactive molecule, but not IL-12 p70.Immunology. 2009 Jul;127(3):312-25. doi: 10.1111/j.1365-2567.2008.02985.x. Epub 2008 Nov 14. Immunology. 2009. PMID: 19019087 Free PMC article.
-
Induction of IL-2 by interleukin-12 p40 homodimer and IL-12, but not IL-23, in microglia and macrophages: Implications for multiple sclerosis.Cytokine. 2024 Feb;174:156457. doi: 10.1016/j.cyto.2023.156457. Epub 2023 Dec 5. Cytokine. 2024. PMID: 38056248 Free PMC article.
-
IL-12 p40 monomer is different from other IL-12 family members to selectively inhibit IL-12Rβ1 internalization and suppress EAE.Proc Natl Acad Sci U S A. 2020 Sep 1;117(35):21557-21567. doi: 10.1073/pnas.2000653117. Epub 2020 Aug 19. Proc Natl Acad Sci U S A. 2020. PMID: 32817415 Free PMC article.
-
The power of combinatorial immunology: IL-12 and IL-12-related dimeric cytokines in infectious diseases.Med Microbiol Immunol. 2004 Feb;193(1):1-17. doi: 10.1007/s00430-003-0186-x. Epub 2003 Jun 27. Med Microbiol Immunol. 2004. PMID: 12836019 Review.
Cited by
-
Trajectory of inflammatory and microglial activation markers in the postnatal rabbit brain following intrauterine endotoxin exposure.Neurobiol Dis. 2018 Mar;111:153-162. doi: 10.1016/j.nbd.2017.12.013. Epub 2017 Dec 21. Neurobiol Dis. 2018. PMID: 29274431 Free PMC article.
-
Increased Levels of IL-16 in the Central Nervous System during Neuroinflammation Are Associated with Infiltrating Immune Cells and Resident Glial Cells.Biology (Basel). 2021 May 27;10(6):472. doi: 10.3390/biology10060472. Biology (Basel). 2021. PMID: 34071825 Free PMC article.
-
Is There a Link between Basal Metabolic Rate, Spleen Volume and Hepatic Growth Factor Levels in Patients with Obesity-Related NAFLD?J Clin Med. 2019 Sep 20;8(10):1510. doi: 10.3390/jcm8101510. J Clin Med. 2019. PMID: 31547124 Free PMC article.
-
Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide.J Neuroinflammation. 2011 Oct 13;8:139. doi: 10.1186/1742-2094-8-139. J Neuroinflammation. 2011. PMID: 21995440 Free PMC article.
-
Attenuation of microglial RANTES by NEMO-binding domain peptide inhibits the infiltration of CD8(+) T cells in the nigra of hemiparkinsonian monkey.Neuroscience. 2015 Aug 27;302:36-46. doi: 10.1016/j.neuroscience.2015.03.011. Epub 2015 Mar 14. Neuroscience. 2015. PMID: 25783477 Free PMC article.
References
-
- Bright JJ, Musuro BF, Du C, Sriram S. Expression of IL-12 in CNS and lymphoid organs of mice with experimental allergic encephalitis. J. Neuroimmunol. 1998;82:22–30. - PubMed
-
- Constantinescu CS, Goodman DB, Hilliard B, Wysocka M, Cohen JA. Murine macrophages stimulated with central and peripheral nervous system myelin or purified myelin proteins release inflammatory products. Neurosci. Lett. 2000;287:171–174. - PubMed
-
- Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. - PubMed
-
- Cruikshank WW, Kornfeld H, Center DM. Signaling and functional properties of interleukin-16. Int. Rev. Immunol. 1998;16:523–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

