CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity
- PMID: 19100702
- PMCID: PMC2757129
- DOI: 10.1016/j.immuni.2008.10.015
CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity
Abstract
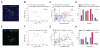
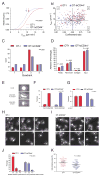
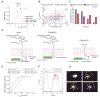
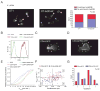
Although T lymphocytes are constitutively nonadherent cells, they undergo facultative polarity during migration and upon interaction with cells presenting cognate antigen, suggesting that cell polarity might be critical for target cell destruction. Using two-photon imaging of tumor-infiltrating T lymphocytes, we found that CD44, a receptor for extracellular matrix proteins and glycosaminoglycans, was crucial for interstitial T cell navigation and, consequently, efficient tumor cell screening. CD44 functioned as a critical regulator of intratumoral movement by stabilizing cell polarity in migrating T cells, but not during target cell interactions. Stable anterior-posterior asymmetry was maintained by CD44 independently of its extracellular domain. Instead, migratory polarity depended on the recruitment of ezrin, radixin, moesin (ERM) proteins by the intracellular domain of CD44 to the posterior cellular protrusion. Our results formally demonstrate that CD44-dependent T lymphocyte locomotion within target sites represents an essential immunologic checkpoint that determines the potency of T cell effector functions.
Figures


Comment in
-
CD44 keeps tumor killers polarized.Immunity. 2008 Dec 19;29(6):843-5. doi: 10.1016/j.immuni.2008.12.004. Immunity. 2008. PMID: 19100697
References
-
- Alstergren P, Zhu B, Glogauer M, Mak TW, Ellen RP, Sodek J. Polarization and directed migration of murine neutrophils is dependent on cell surface expression of CD44. Cell Immunol. 2004;231:146–157. - PubMed
-
- Bousso P, Robey EA. Dynamic behavior of T cells and thymocytes in lymphoid organs as revealed by two-photon microscopy. Immunity. 2004;21:349–355. - PubMed
-
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

