Wnt5a is essential for intestinal elongation in mice
- PMID: 19100728
- PMCID: PMC2654720
- DOI: 10.1016/j.ydbio.2008.11.020
Wnt5a is essential for intestinal elongation in mice
Abstract
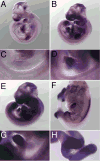
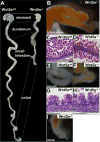
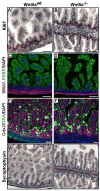
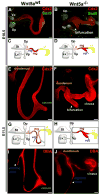
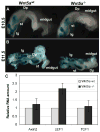
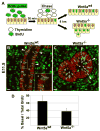
Morphogenesis of the mammalian small intestine entails extensive elongation and folding of the primitive gut into a tightly coiled digestive tube. Surprisingly, little is known about the cellular and molecular mechanisms that mediate the morphological aspects of small intestine formation. Here, we demonstrate that Wnt5a, a member of the Wnt family of secreted proteins, is essential for the development and elongation of the small intestine from the midgut region. We found that the small intestine in mice lacking Wnt5a was dramatically shortened and duplicated, forming a bifurcated lumen instead of a single tube. In addition, cell proliferation was reduced and re-intercalation of post-mitotic cells into the elongating gut tube epithelium was disrupted. Thus, our study demonstrates that Wnt5a functions as a critical regulator of midgut formation and morphogenesis in mammals.
Figures


References
-
- Ahnfelt-Rønne J, Jørgensen MC, Hald H, Madsen OD, Serup P, Hecksher-Sørensen J. An improved method for three-dimensional reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J Histochem Cytochem. 2007;55:925–30. - PubMed
-
- Brandvold KA, Ewert DL, Kent SC, Neiman P, Ruddell A. Blocked B cell differentiation and emigration support the early growth of Myc-induced lymphomas. Oncogene. 2001;20:3226–34. - PubMed
-
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

