Analysis of flavivirus NS5 methyltransferase cap binding
- PMID: 19101564
- PMCID: PMC2680092
- DOI: 10.1016/j.jmb.2008.11.058
Analysis of flavivirus NS5 methyltransferase cap binding
Abstract
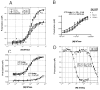
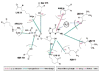
The flavivirus 2'-O-nucleoside N-terminal RNA methyltransferase (MTase) enzyme is responsible for methylating the viral RNA cap structure. To increase our understanding of the mechanism of viral RNA cap binding we performed a detailed structural and biochemical characterization of the guanosine cap-binding pocket of the dengue (DEN) and yellow fever (YF) virus MTase enzymes. We solved an improved 2.1 A resolution crystal structure of DEN2 Mtase, new 1.5 A resolution crystal structures of the YF virus MTase domain in apo form, and a new 1.45 A structure in complex with guanosine triphosphate and RNA cap analog. Our structures clarify the previously reported DEN MTase structure, suggest novel protein-cap interactions, and provide a detailed view of guanine specificity. Furthermore, the structures of the DEN and YF proteins are essentially identical, indicating a large degree of structural conservation amongst the flavivirus MTases. Guanosine triphosphate analog competition assays and mutagenesis analysis, performed to analyze the biochemical characteristics of cap binding, determined that the major interaction points are (i) guanine ring via pi-pi stacking with Phe24, N1 hydrogen interaction with the Leu19 backbone carbonyl via a water bridge, and C2 amine interaction with Leu16 and Leu19 backbone carbonyls; (ii) ribose 2' hydroxyl interaction with Lys13 and Asn17; and (iii) alpha-phosphate interactions with Lys28 and Ser215. Based on our mutational and analog studies, the guanine ring and alpha-phosphate interactions provide most of the energy for cap binding, while the combination of the water bridge between the guanine N1 and Leu19 carbonyl and the hydrogen bonds between the C2 amine and Leu16/Leu19 carbonyl groups provide for specific guanine recognition. A detailed model of how the flavivirus MTase protein binds RNA cap structures is presented.
Figures



References
-
- Lindenbach BD, Rice CM. Flaviviridae: The Viruses and Their Replication. In: Knipe DM, Howley P, editors. Fields Virology. 4. Vol. 1. Lippincott, Williams, and Wilkins; 2000. pp. 991–1041. 2 vols.
-
- Yon C, Teramoto T, Mueller N, Phelan J, Ganesh VK, Murthy KH, Padmanabhan R. Modulation of the nucleoside triphosphatase/RNA helicase and 5′-RNA triphosphatase activities of Dengue virus type 2 nonstructural protein 3 (NS3) by interaction with NS5, the RNA-dependent RNA polymerase. J Biol Chem. 2005;280:27412–9. - PubMed
-
- Kuo MD, Chin C, Hsu SL, Shiao JY, Wang TM, Lin JH. Characterization of the NTPase activity of Japanese encephalitis virus NS3 protein. J Gen Virol. 1996;77(Pt 9):2077–84. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

