Conserved sequence in the aggrecan interglobular domain modulates cleavage by ADAMTS-4 and ADAMTS-5
- PMID: 19101611
- PMCID: PMC2656578
- DOI: 10.1016/j.bbagen.2008.11.008
Conserved sequence in the aggrecan interglobular domain modulates cleavage by ADAMTS-4 and ADAMTS-5
Abstract
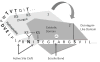
Background: Cleavage of aggrecan by ADAMTS proteinases at specific sites within highly conserved regions may be important to normal physiological enzyme functions, as well as pathological degradation.
Methods: To examine ADAMTS selectivity, we assayed ADAMTS-4 and -5 cleavage of recombinant bovine aggrecan mutated at amino acids N-terminal or C-terminal to the interglobular domain cleavage site.
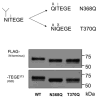
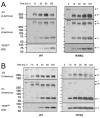
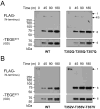
Results: Mutations of conserved amino acids from P18 to P12 to increase hydrophilicity resulted in ADAMTS-4 cleavage inhibition. Mutation of Thr, but not Asn within the conserved N-glycosylation motif Asn-Ile-Thr from P6 to P4 enhanced cleavage. Mutation of conserved Thr residues from P22 to P17 to increase hydrophobicity enhanced ADAMTS-4 cleavage. A P4' Ser377Gln mutant inhibited cleavage by ADAMTS-4 and -5, while a neutral Ser377Ala mutant and species mimicking mutants Ser377Thr, Ser377Asn, and Arg375Leu were cleaved normally by ADAMTS-4. The Ser377Thr mutant, however, was resistant to cleavage by ADAMTS-5.
Conclusion: We have identified multiple conserved amino acids within regions N- and C-terminal to the site of scission that may influence enzyme-substrate recognition, and may interact with exosites on ADAMTS-4 and ADAMTS-5.
General significance: Inhibition of the binding of ADAMTS-4 and ADAMTS-5 exosites to aggrecan should be explored as a therapeutic intervention for osteoarthritis.
Figures
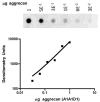
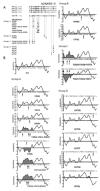
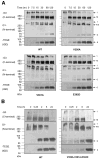
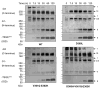

Similar articles
-
ADAMTS-4_v1 is a splice variant of ADAMTS-4 that is expressed as a protein in human synovium and cleaves aggrecan at the interglobular domain.Arthritis Rheum. 2013 Nov;65(11):2866-75. doi: 10.1002/art.38102. Arthritis Rheum. 2013. PMID: 23897278 Free PMC article.
-
ADAMTS-5 deficiency does not block aggrecanolysis at preferred cleavage sites in the chondroitin sulfate-rich region of aggrecan.J Biol Chem. 2007 Mar 23;282(12):8632-40. doi: 10.1074/jbc.M605750200. Epub 2007 Jan 25. J Biol Chem. 2007. PMID: 17255106
-
Regulation of aggrecanases from the ADAMTS family and aggrecan neoepitope formation during in vitro chondrogenesis of human mesenchymal stem cells.Eur Cell Mater. 2012 May 4;23:320-32. doi: 10.22203/ecm.v023a25. Eur Cell Mater. 2012. PMID: 22562232
-
Aggrecanase and aggrecan degradation in osteoarthritis: a review.J Int Med Res. 2008 Nov-Dec;36(6):1149-60. doi: 10.1177/147323000803600601. J Int Med Res. 2008. PMID: 19094423 Review.
-
ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis.J Cell Biochem. 2011 Dec;112(12):3507-14. doi: 10.1002/jcb.23298. J Cell Biochem. 2011. PMID: 21815191 Review.
Cited by
-
The perineuronal net component of the extracellular matrix in plasticity and epilepsy.Neurochem Int. 2012 Dec;61(7):963-72. doi: 10.1016/j.neuint.2012.08.007. Epub 2012 Aug 29. Neurochem Int. 2012. PMID: 22954428 Free PMC article. Review.
-
ADAMTS proteases: key roles in atherosclerosis?J Mol Med (Berl). 2010 Dec;88(12):1203-11. doi: 10.1007/s00109-010-0654-x. Epub 2010 Jul 22. J Mol Med (Berl). 2010. PMID: 20652528 Review.
-
Development of a fluorogenic ADAMTS-7 substrate.J Enzyme Inhib Med Chem. 2021 Dec;36(1):2160-2169. doi: 10.1080/14756366.2021.1983808. J Enzyme Inhib Med Chem. 2021. PMID: 34587841 Free PMC article.
-
Comparison of sensory neuron growth cone and filopodial responses to structurally diverse aggrecan variants, in vitro.Exp Neurol. 2013 Sep;247:143-57. doi: 10.1016/j.expneurol.2013.02.012. Epub 2013 Mar 1. Exp Neurol. 2013. PMID: 23458191 Free PMC article.
References
-
- Hughes CE, Caterson B, Little DB, Wainwright SW. Aggrecanases 1 and 2. In: RN, Barrett AJ, Woesneer JF, editors. Handbook of proteolytic enzymes. Elsevier Academic Press; London: 2004. pp. 740–746.
-
- Lee ER, Lamplugh L, Davoli MA, Beauchemin A, Chan K, Mort JS, Leblond CP. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats ages 0–21 days: I. Two groups of proteinases cleave the core protein of aggrecan. Dev Dyn. 2001;222:52–70. - PubMed
-
- Lee ER, Lamplugh L, Leblond CP, Mordier S, Magny MC, Mort JS. Immunolocalization of the cleavage of the aggrecan core protein at the Asn341-Phe342 bond, as an indicator of the location of the metalloproteinases active in the lysis of the rat growth plate. Anat Rec. 1998;252:117–132. - PubMed
-
- Mort JS, Flannery CR, Makkerh J, Krupa JC, Lee ER. Use of anti-neoepitope antibodies for the analysis of degradative events in cartilage and the molecular basis for neoepitope specificity. Biochem Soc Symp. 2003:107–114. - PubMed
-
- Bayliss MT, Hutton S, Hayward J, Maciewicz RA. Distribution of aggrecanase (ADAMts 4/5) cleavage products in normal and osteoarthritic human articular cartilage: the influence of age, topography and zone of tissue. Osteoarthritis Cartilage. 2001;9:553–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

