Hexokinase-2 bound to mitochondria: cancer's stygian link to the "Warburg Effect" and a pivotal target for effective therapy
- PMID: 19101634
- PMCID: PMC2714668
- DOI: 10.1016/j.semcancer.2008.11.006
Hexokinase-2 bound to mitochondria: cancer's stygian link to the "Warburg Effect" and a pivotal target for effective therapy
Abstract
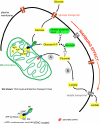
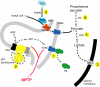
The most common metabolic hallmark of malignant tumors, i.e., the "Warburg effect" is their propensity to metabolize glucose to lactic acid at a high rate even in the presence of oxygen. The pivotal player in this frequent cancer phenotype is mitochondrial-bound hexokinase [Bustamante E, Pedersen PL. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc Natl Acad Sci USA 1977;74(9):3735-9; Bustamante E, Morris HP, Pedersen PL. Energy metabolism of tumor cells. Requirement for a form of hexokinase with a propensity for mitochondrial binding. J Biol Chem 1981;256(16):8699-704]. Now, in clinics worldwide this prominent phenotype forms the basis of one of the most common detection systems for cancer, i.e., positron emission tomography (PET). Significantly, HK-2 is the major bound hexokinase isoform expressed in cancers that exhibit a "Warburg effect". This includes most cancers that metastasize and kill their human host. By stationing itself on the outer mitochondrial membrane, HK-2 also helps immortalize cancer cells, escapes product inhibition and gains preferential access to newly synthesized ATP for phosphorylating glucose. The latter event traps this essential nutrient inside the tumor cells as glucose-6-P, some of which is funneled off to serve as carbon precursors to help promote the production of new cancer cells while much is converted to lactic acid that exits the cells. The resultant acidity likely wards off an immune response while preparing surrounding tissues for invasion. With the re-emergence and acceptance of both the "Warburg effect" as a prominent phenotype of most clinical cancers, and "metabolic targeting" as a rational therapeutic strategy, a number of laboratories are focusing on metabolite entry or exit steps. One remarkable success story [Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun 2004;324(1):269-75] is the use of the small molecule 3-bromopyruvate (3-BP) that selectively enters and destroys the cells of large tumors in animals by targeting both HK-2 and the mitochondrial ATP synthasome. This leads to very rapid ATP depletion and tumor destruction without harm to the animals. This review focuses on the multiple roles played by HK-2 in cancer and its potential as a metabolic target for complete cancer destruction.
Figures
References
-
- Wilson JE. Hexokinases. Rev Physiol Biochem Pharmacol. 1995;126:65–198. - PubMed
-
- Wilson JE. An introduction to the isoenzymes of mammalian hexokinase types I-III. Biochem Soc Trans. 1997;25:103–7. - PubMed
-
- Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–57. - PubMed
-
- Tsai HJ, Wilson JE. Functional organization of mammalian hexokinases: characterization of chimeric hexokinases constructed from the N- and C-terminal domains of the rat type I and type II isozymes. Arch Biochem Biophys. 1995;316:206–14. - PubMed
-
- Tsai HJ, Wilson JE. Functional organization of mammalian hexokinases: both N- and C-terminal halves of the rat type II isozyme possess catalytic sites. Arch Biochem Biophys. 1996;329:17–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

